Jun 23 2016
In the last century, a 20-sided solid was morphed into geodesic domes. This solid is likely to play a major role in synthetic biology. The geometry of the icosahedron is inspiring a team of researchers from the University of Washington Institute of Protein Design who are involved in inventing vehicles, molecular tools, and devices for medicine and other fields.
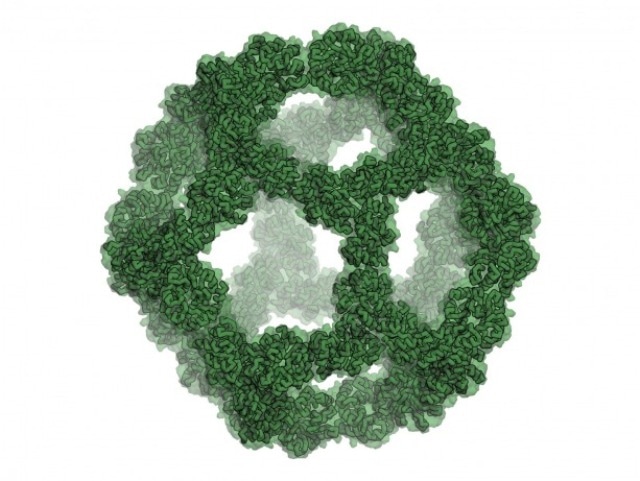 The design model of the icosahedral nano-cage shows its large, empty volume. (Photo credit: UW Institute for Protein Design)
The design model of the icosahedral nano-cage shows its large, empty volume. (Photo credit: UW Institute for Protein Design)
The icosahedron possesses bird cage-like symmetry and large interior indicating cargo-containing probabilities. The team took their cue from the several viruses that, on the way to living cells, convey their genomes inside protective icosahedral protein shells.
These delivery packages are called viral capsids, and are built to be adequately strong to handle the travel, efficiently utilize storage room, and break separately to emit their contents when conditions are appropriate.
The researchers have published a paper in the scientific journal Nature. It illustrates their computational design and experimental testing of an extremely stable icosahedral protein nano-cage. This nano-cage, engineered at the atomic level, can form by itself from data encoded in strands of DNA and biochemical building blocks.
After choosing the design for this icosahedral nano-cage using computer modeling, the team generated it in bacteria. Electron microscopy of the ensuing icosahedral particles verified that they were almost matching to the design model.
“The ability to design proteins that self-assemble into precisely specified, robust, and highly order icosahedral structures,” the researchers wrote, “would open the door to a new generation of protein containers with properties custom-made for applications of interest.”
It is possible that fabricating nanoscale icosahedral vehicles could be among these applications. This type of research has potential for the creation of miniature spacecraft-like devices capable of encapsulating and delivering therapies directly to particular types of cells, such as cancer cells.
Although strong, the designed icosahedron could disassemble and reassemble itself under specific environmental conditions. This reversible feature is necessary if it ultimately becomes part of packaging, carrying and supplying a biochemical agent. The researchers feel that in addition to possessing flexibility to modify these miniature cages, it “should have considerable utility for targeted drug delivery, vaccine design and synthetic biology.”
The recently designed icosahedron has significantly larger internal volume than formerly designed nano-cages possessing other shapes, and therefore could contain extra cargo as molecular shipping cases. The team began focusing on this aspect, and were able to devise barriers for the center of all the 20 faces of the icosahedron.
These would possess the ability to block molecules from flowing in and out of the cage. Going forward, future iterations may have gated cages that could be filled to covey a medication into specific types of cell and then release it.
Furthermore, the protein building blocks constituting the cage maintain their natural enzymatic action, which is the ability to accelerate chemical reactions. This proposes the possibility of custom designing them as nano-reactors to catalyze particular biochemical procedures. Besides, the nano-cages were agreeable to genetic fusions to improve their properties.
For instance, the team developed standard candles for light microscopy. They did this by adding a fluorescent protein to all of the 60 subunits that frame the icosahedron. The fluorescent intensity was relative to the number of these proteins fastened to each subunit. The icosahedron’s unique shape makes it an easily spotted marker.
The project leads were Yang Hsia, a University of Washington graduate student in biological physics, structure and design, and Jacob B. Bale, a recent graduate from the UW molecular and cellular biology Ph.D. program, and now a research scientist at Arzeda Corporation in Seattle. The senior authors were Neil P. King, translational investigator at the UW Institute for Protein Design, and David Baker, director of the Institute and UW professor of biochemistry. Baker is also an investigator with the Howard Hughes Medical Institute.
The research project was supported by the Howard Hughes Medical Institute, the National Science Foundation, the Bill & Melinda Gates Foundation, a University of Washington/Fred Hutchinson Cancer Research Institute Pilot Award from the National Cancer Institute, the National Institutes of Health, the JRC Visitor Program, the Takeda Pharmaceutical Company, and a Public Health Services National Research Services Award.