Oct 27 2017
Scientists from Russia have devised a mechanism which involves using green light to detect molecular hydrogen in order to light up a nanocrystalline composite sensor based on indium and zinc oxides. This is the first time that a gas sensor has been able to function at ambient temperature. The study was reported in the Scientific Reports journal.
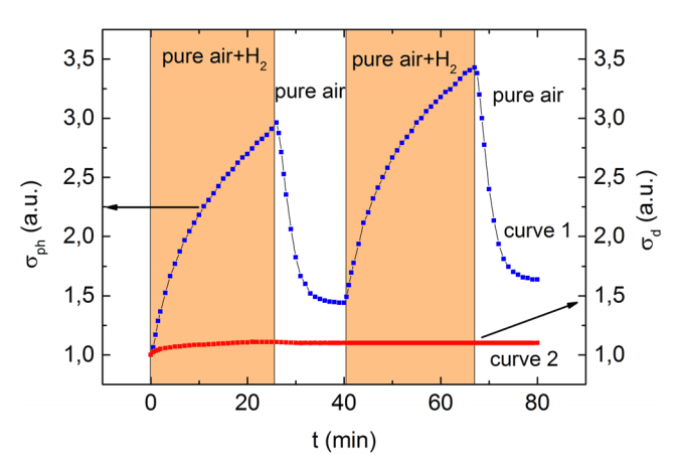 Photoconductivity (curve 1) and electrical conductivity (curve 2) of a composite film made of zinc and indium oxides in a 1-to-9 ratio by weight under periodic exposure to air containing hydrogen. Orange marks the scope of sensor response. Credit: Moscow Institute of Physics and Technology
Photoconductivity (curve 1) and electrical conductivity (curve 2) of a composite film made of zinc and indium oxides in a 1-to-9 ratio by weight under periodic exposure to air containing hydrogen. Orange marks the scope of sensor response. Credit: Moscow Institute of Physics and Technology
At present, multisensor arrays for ascertaining the composition of gas mixtures are being designed. They are monitoring systems including a number of sensors targeting distinctive gases. The sensors can be applied to investigate air quality outdoors as well as indoors. Recording atmospheric pollution is a significant task for a number of developed countries. Due to the fact that residential communities are normally concentrated around industrial areas, it is obligatory to have a process in place for regulating hazardous emissions from factories and plants.
Apart from that, evaluations of air composition are mandatory on space stations and submarines, near nuclear power plants, and at other places where fresh air is not instantly accessible: in the event of leakage of a toxic chemical into the ventilation system or increase in the concentration of carbon dioxide, this may pose danger to the lives of workers.
The composition of commercial gas mixtures (e.g. gas fuels such as hydrogen) should also be accurately monitored. When used as a gas fuel, hydrogen has the ability to prospectively be a substitute for hydrocarbons. Hydrogen is a clean fuel that emits only water vapor during combustion. Moreover, when compared to hydrocarbons, the efficiency of hydrogen combustion is 10-20% greater. Certain car manufacturers have already started to introduce hydrogen as a futuristic fuel. However, the Hindenburg airship tragedy sadly shows the risks of using hydrogen as a fuel.
Until recently, the operating temperatures of gas sensors that operated on nanocrystalline metal oxides were 300-500 °C, which rendered them unreliable to be used for detecting combustible or explosive substances. Furthermore, immense power is needed to maintain such high temperatures, a lot of poweris required, making it impossible to embed such gas sensors into the circuit boards of portable devices.
In order to overcome this difficulty, Professor Leonid Trakhtenberg from MIPT; Pavel Kashkarov, Director of the Institute of Nano-, Bio-, Information, Cognitive and Socio-Humanistic Science and Technology; Alexander Ilin and Pavel Forsh of Lomonosov Moscow State University; and their collaborators from Semenov Institute of Chemical Physics suggested using sensors than can function at ambient temperature. The innovative nanocomposite sensors employed by the Researchers are designed using indium and zinc oxides, and their efficaciousness is enhanced by illumination with green light. The device put forward by them can be applied for detecting explosive, combustible, as well as toxic substances in the atmosphere even at lower concentrations.
The mechanism consists in the light-induced transition of the nanocrystalline sensor components into a nonequilibrium state and the resulting change in the photoconductivity of the sensor interacting with molecular hydrogen. This effect is linked with the dependence of photoconductivity on the nonequilibrium charge carrier recombination rate.
Maria Ikim, Doctoral Student, The Laboratory of Functional Nanocomposites, Semenov Institute of Chemical Physics, The Russian Academy of Sciences
“The detectors that we have developed differ from the conventional semiconductor sensors in that they operate at room temperature. This eliminates the danger of combustion or explosion, when flammable or explosive substances are involved,” stated Leonid Trakhtenberg from the Department of Chemical Physics, MIPT, who holds an ScD in Physics and Mathematics. “Most papers on sensor photoactivation discuss the effects of ultraviolet light on sensors and focus on the detection of oxidizing gases. But the efficiency of ultraviolet light diodes is low, while their cost is far greater than that of their counterparts emitting in the visible part of the spectrum. By working with hydrogen, we explore the possibilities of the detection of reducing gases.”
The study described here puts forward an innovative mechanism of sensor response photoactivation. It takes into account the changeover of charge carriers into a state of nonequilibrium. The mechanism adopted is universal: It can be applied to understand sensing outcomes in reducing as well as oxidizing gases.
The sensors developed by the Researchers can be used to record atmospheric air composition and investigate the chemical composition of gases adopted for industrial procedures. Despite the fact that the research concentrates around gases, the same sensors can be altered to be applied for liquids.