Aug 27 2018
Engineers from the Rice University have discovered a way to fit a square peg into a round hole. The first step is to obtain a nanotube hole and the next is to insert water. If the nanotube has the correct width, the water molecules will align into a square rod.
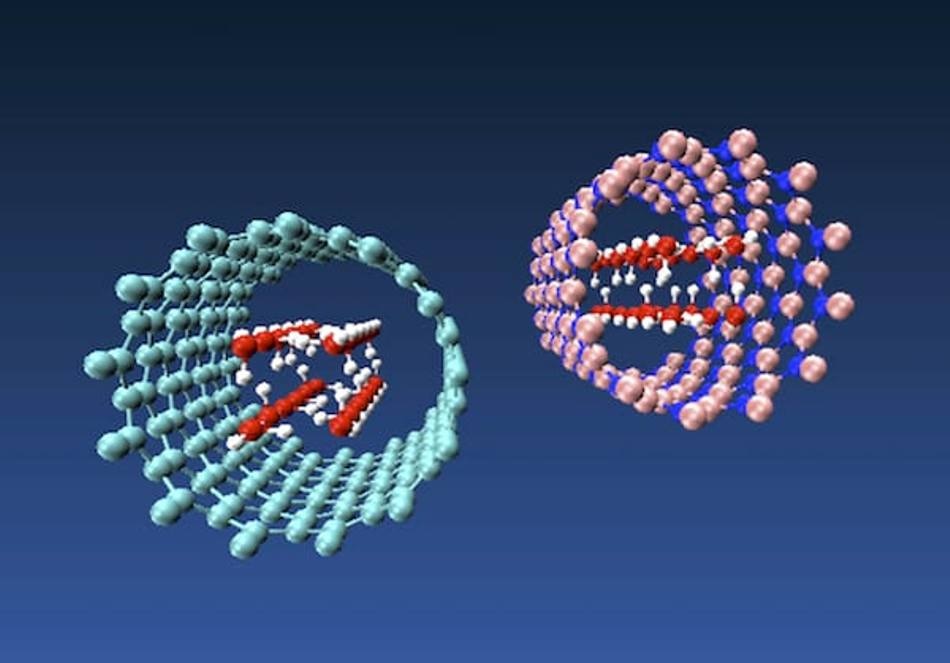 Molecular models of nanotube ice produced by engineers at Rice University show how forces inside a carbon nanotube at left and a boron nitride nanotube at right pressure water molecules into taking on the shape of a square tube. The phenomenon is dependent upon the diameter of the nanotube. (Image credit: Multiscale Materials Laboratory)
Molecular models of nanotube ice produced by engineers at Rice University show how forces inside a carbon nanotube at left and a boron nitride nanotube at right pressure water molecules into taking on the shape of a square tube. The phenomenon is dependent upon the diameter of the nanotube. (Image credit: Multiscale Materials Laboratory)
Rouzbeh Shahsavari, a Rice materials scientist, and his colleagues employed molecular models to demonstrate their theory that the strength of the weak van der Waals forces between the water molecules and the inner surface of the nanotube is adequate enough to snap the oxygen and hydrogen atoms into place.
Shahsavari mentioned the contents as two-dimensional “ice” since the molecules freeze irrespective of the temperature. He stated that the study offers a valuable understanding of methods to leverage atomic interactions between water molecules and nanotubes to develop nanochannels and energy-storing nanocapacitors.
A paper describing the study appears in Langmuir, an American Chemical Society journal.
Shahsavari and his team developed molecular models of carbon and boron nitride nanotubes with modifiable widths. They found out that boron nitride is best at restricting the shape of water when the width of the nanotubes is 10.5 Angstroms, where 1 Angstrom is equal to 100-millionth of 1 cm.
The scientists were already aware that hydrogen atoms in tightly constrained water take on intriguing structural characteristics. Latest experiments performed in other labs demonstrated strong evidence for the synthesis of nanotube ice and induced the scientists to develop density functional theory models for the analysis of the forces responsible.
Shahsavari and his colleagues modeled water molecules, with a width of around 3 Angstroms, inside carbon and boron nitride nanotubes of differing chiralities (the angles of their atomic lattices) and with a diameter of 8–12 Angstroms. They found out that nanotubes in the middle diameters had the highest effect on the balance between van der Waals pressure and molecular interactions that induced the transition from a square water tube to ice.
If the nanotube is too small and you can only fit one water molecule, you can’t judge much. If it’s too large, the water keeps its amorphous shape. But at about 8 angstroms, the nanotubes’ van der Waals force starts to push water molecules into organized square shapes.
Rouzbeh Shahsavari
He stated that the strongest interactions were discovered in boron nitride nanotubes owing to the specific polarization of their atoms.
Shahsavari told that nanotube ice could be applied as nanoscale capillaries or in molecular machines, or promote techniques for delivering a few molecules of sequestered drugs or water to targeted cells, such as a nanoscale syringe.
Farzaneh Shayeganfar, the lead author of the study who is a former visiting scholar at Rice, is an instructor at Shahid Rajaee Teacher Training University in Tehran, Iran. Javad Beheshtian, co-principal investigator of the study, is a professor at Amirkabir University, Tehran.
Supercomputer resources were provided with support from the National Institutes of Health and an IBM Shared Rice University Research grant.