Water destined for distribution into the public water system is extracted from a number of sources. Each of these sources needs to be treated in a specific way to remove suspended material, viruses and bacteria and humic acids which colour the water.

Image Credits: mr.water/shutterstock.com
EEC drinking water regulations applied in 1989 and higher expectations of water quality worldwide mean that traditional methods of treatment and control are inadequate to ensure that recommended contaminant levels are never exceeded.
Water clarity is measured by monitoring the turbidity. This is useful to check the effectiveness of the treatment process, but is no use in controlling dosing or investigating the source of a problem if one arises.
The Answer
New control processes are needed which rely on the fundamental parameter controlling flocculation, the zeta potential. The Malvern Panalytical Zetasizer makes the measurement of zeta potential simple, fast and reproducible. A typical water treatment plant is shown in fig. 1. The process involves altering the pH, adding salts or polyelectrolytes, or a combination of these. This flocculates the contaminants which will then sediment or are filtered out.
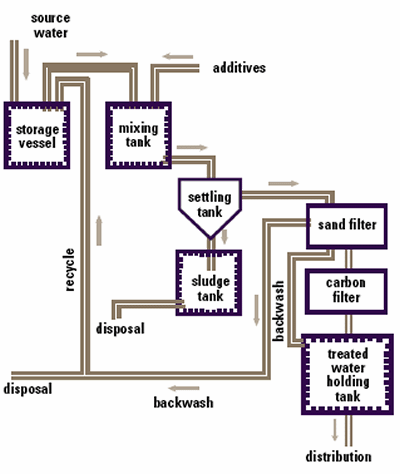
Figure 1. Schematic of a typical water treatment system.
The Theory of Water Treatment
These treatments work by altering the zeta potential of the suspended material. Maximizing the efficiency of additive addition and control of flocculation can be done by routine measurement of the zeta potential. To destabilize a suspension or increase the rate of flocculation the zeta potential must be reduced.
Zeta Potential
The zeta potential is the parameter that determines the electrical interaction between particles, a high value prevents flocculation, reducing the value allows particles to approach each other and flocculate (fig. 2), the fastest rate of flocculation being the point of zero zeta potential (pzzp). The rate of flocculation can also be associated with the compactness of the floc which affects the rate of filtration.
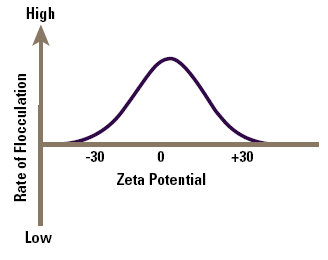
Figure 2. How zeta potential affects flocculation.
The DLVO Theory
The theoretical basis for the concept of zeta potential is described by the DLVO theory.
This demonstrates how colloid stability is determined by the balance between the Van der Waals attractive forces and electrical repulsive forces (zeta potential) between particles. Plotting zeta potential over a range of electrolyte concentrations allows the critical coagulation concentration (CCC) to be determined. Below this value the suspended material will not flocculate but may sediment slowly, forming a dense deposit. Above the CCC there will be flocculation; the material will sediment more quickly and form a lower density deposit.
Contamination
Mineral oxides form a significant proportion of the suspended material in ground water. The point of zero zeta potential (pzzp) for many mineral oxides is between 7 and 9 and H+, OH- are potential determining ions. This means that pH will be one of the main factors determining zeta potential.
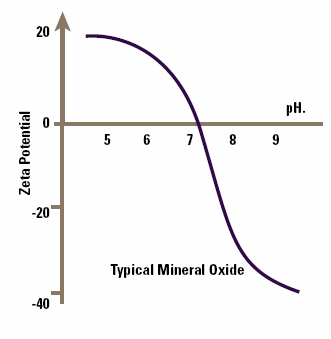
Figure 3. The effect of pH on zeta potential for mineral oxides.
The Effect of Different Treatments
pH
Source water will be typically pH5 to 6, adding alkali will reduce the zeta potential of most contaminants and increase the rate of flocculation. pH adjustment is not usually used on its own, but in conjunction with other treatments to increase their effectiveness.
Electrolytes
Investigation of the correct pH for use of these ions is essential because the adsorption efficiency of the metal ions onto an oxide surface can vary from 0 – 100% over a pH range of less than 1. Adsorption efficiency is directly related to the reduction in the zeta potential.
Polyelectrolytes
Polyelectrolytes are charged polymers which naturally adsorb onto particle surfaces. The polymer is of opposite charge to the particle so adsorption reduces the zeta potential. The quantity added and the rate of addition both affect the rate of flocculation.
Waste Water
Water used in the paper or mineral processing industries has to be treated before re-use or discharge into rivers etc. The same principles for domestic supply water treatment apply to waste water and ground water contaminated with toxic metals, insecticides and herbicides.
Conclusion
Using the Malvern Panalytical Zetasizer enables the measurement and control of the zeta potential of suspended material in water in a treatment plant. This will improve water quality and increase plant throughput.

This information has been sourced, reviewed and adapted from materials provided by Malvern Panalytical.
For more information please visit Malvern Panalytical.