Apr 20 2020
At LMU, scientists have developed nanoparticles that can be triggered, through a change in pH, to discharge a lethal dose of ionized iron within cells. This mechanism could pave the way for new methods to the targeted removal of malignant tumors.
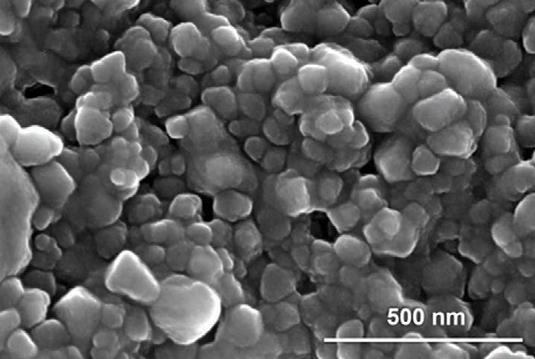 SEM images of lipid-coated MIL-100(Fe) (e) nanoparticles at 150,000× magnification. Image Credit: Ploetz et al., Advanced Materials 2020.
SEM images of lipid-coated MIL-100(Fe) (e) nanoparticles at 150,000× magnification. Image Credit: Ploetz et al., Advanced Materials 2020.
Ions have critical roles to play in all attributes of cell biology. They control enzyme activities, activate signaling cascades, and regulate the pH of the intra- and extracellular media. Thus, the free ion concentrations are strongly controlled, and abrupt variations in their intracellular levels can lead to programmed cell death.
But this fact alone has rendered it challenging to determine the complex mechanisms that regulate the concentrations of ions in cells. Since cells respond quickly to inhibit excess ion import, they effectively confront attempts to exploit intracellular ion levels.
At present, a team of researchers headed by Hanna Engelke and Evelyn Ploetz (Faculty of Chemistry and Pharmacy, LMU) has developed nanoparticles that enable, for the first time, quick and controlled activation of the large-scale release of ionic iron inside cells. This, in turn, leads to a kind of inflammatory cell death called pyroptosis, a reaction that is intrinsic to cells of the inherent immune system.
The new study has been published in the Advanced Materials journal and describes that the potential to trigger pyroptosis as needed could be leveraged to remove malignant cells, as well as to induce an immune reaction that is directed particularly against cancers.
The rapid-release effect is a direct outcome of the structural characteristics of the nanoparticles, which are part of a family of substances called metal-organic frameworks (MOFs). The interstices that these frameworks form offer identical binding sites to which other substances—here, iron-oxygen complexes—can particularly bind.
Structurally, these binding sites are tiny hexagons that are connected to each other by organic linker molecules. MOFs can be thought of as scaffolds, and the pores within each nanoparticle are large enough to allow reaction partners to diffuse into them.
Evelyn Ploetz, Faculty of Chemistry and Pharmacy, LMU
Furthermore, lipids coated on the nanoparticles allow them to be absorbed by cells. As soon as they enter the cell, the nanoparticles are transported into organelles known as lysosomes, where they are disintegrated.
“We were able to demonstrate that the rate of degradation depends on the pH of the extracellular medium. If the pH value is relatively low, as it is in an acidic milieu, degradation occurs rapidly, which results in a sudden and massive release of iron ions,” added Ploetz.
Ploetz and her team doubt that this effect could be because, under slightly acidic conditions, cysteine—the reduced form of the amino acid that supports the dissolution of the nanoparticles—is present in excess.
We were particularly surprised to find that the release of iron from the nanoparticles did not induce ferroptosis, as one might expect in the presence of excess iron. Instead, they trigger a reaction known as pyroptosis.
Evelyn Ploetz, Faculty of Chemistry and Pharmacy, LMU
Induction of pyroptosis in cells of the intrinsic immune system leads to a potent inflammatory reaction, which destroys the relevant cell, but may act as a signal that triggers anti-tumor immunity.
The researchers emphasized that these nanoparticles are highly potent as therapeutic agents, specifically to treat malignant tumors.
The extracellular medium within tumors is more acidic than that associated with normal cells. In principle, this pH difference could be exploited for the targeted release of the iron within the tumor environment. That would enable the nanoparticles to attack the primary tumor directly, while inducing pyroptosis to activate the immune system. But because their properties can be readily controlled by altering the pH, they are also ideally suited for application in other contexts.
Evelyn Ploetz, Faculty of Chemistry and Pharmacy, LMU
Source: https://www.en.uni-muenchen.de/index.html