Dec 4 2020
The fact that carbon nanotubes exhibit fluorescence is well known. However, it is surprising and potentially useful to find a second level of fluorescence. A new study has now revealed how that works.
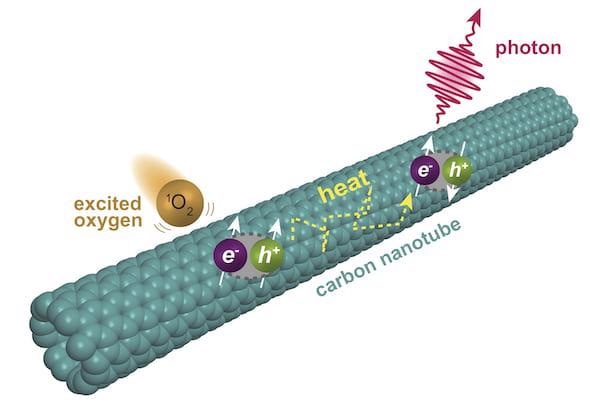 Chemists at Rice University have discovered a second level of fluorescence in single-walled carbon nanotubes. The fluorescence is triggered when oxygen molecules excited into a singlet state interact with nanotubes, prompting excitons to form triplet states that upconvert into fluorescing singlets. Image Credit: Ching-Wei Lin.
Chemists at Rice University have discovered a second level of fluorescence in single-walled carbon nanotubes. The fluorescence is triggered when oxygen molecules excited into a singlet state interact with nanotubes, prompting excitons to form triplet states that upconvert into fluorescing singlets. Image Credit: Ching-Wei Lin.
The Rice University lab of Bruce Weisman, a professor of chemistry who headed the leading discovery of nanotube fluorescence in 2002, identified that single-walled nanotubes radiate a delayed secondary fluorescence upon being activated by a multistep process in a solution consisting of dye molecules and dissolved oxygen.
Although the delay lasts only a few microseconds, it is sufficient to be detected with some effort.
The complex process has been described by Weisman, lead author and Rice alumnus Ching-Wei Lin, and research scientist Sergei Bachilo in the Journal of the American Chemical Society.
The reaction starts when a solution with a dye known as rose bengal is excited by light. Energy from the dye is captured by oxygen molecules dissolved in the solution, thus resulting in energized O2. Then, the energized oxygen molecules transfer their energy to nanotubes, where excitons—quasiparticles formed of electrons and holes—are produced in their triplet state.
With some additional thermal energy, the excitons get excited to a higher energy singlet state that discharges the observed fluorescence.
For a number of years, we have been looking at interesting effects involving nanotubes and oxygen. We’ve found quite a range of things that can happen, from physical effects like this energy transfer or the reversible quenching of fluorescence, to the triggering of chemical reactions between nanotubes and DNA. So this study was part of a larger program of exploration.
Bruce Weisman, Professor of Chemistry, Rice University
The fact that they could excite dissolved oxygen molecules motivated the researchers to analyze how that would influence adjacent nanotubes, added Weisman.
“We make singlet oxygen by exciting a dye molecule with visible light, and then the oxygen deactivates the dye and gets excited itself,” he noted. “That idea goes back decades in photophysics and is very conventional. What’s unusual here is that the singlet oxygen interacts with the nanotube to directly make triplet-state excitations in the tube. Those triplet states have been pretty elusive.”
Triplet states of organic molecules are the longest-lived excited states. Their lifetimes are orders of magnitude greater than the singlet excited states, so they can hang around long enough to bump into something else and undergo chemical reactions.
Bruce Weisman, Professor of Chemistry, Rice University
“But because nanotube triplet states don’t emit light or directly absorb light very well, they are tricky to study and not too much is known about them,” he added. “What we’ve been doing is trying to understand them a little better.”
Activating fluorescence still necessitated an additional step. “Just by random thermal agitation in their surroundings, these guys can sometimes get kicked up to the bright singlet state, and then they can tell you they’re there by spitting out a photon,” stated Weisman.
Since the triplet state can last for about 10 μs, the upconverted emission is termed delayed fluorescence.
The team had to identify a means to observe the comparatively weak effect amid the bright primary fluorescence of the nanotubes. “It was like trying to see a dim object right after being blinded by a bright camera flash,” explained Weisman. “We had to devise some special instrumentation.”
One device “is basically a fast mechanical shutter” that covers the short-wave infrared (SWIR) spectrometer during the bright flash and then opens up rapidly, a type of reverse camera that changes from covered to open in 7 μs.
He added that the other device is a sensitive detector that gets activated with an electronic signal and evaluates how the weak emission fades away over time. “These systems were both built by Ching-Wei, who’s a terrific experimentalist,” he noted.
Weisman and his team have used nanotube fluorescence in nanotube-based smart skin and medical imaging technologies to quantify strain on surfaces, besides other applications. According to him, the new findings could ultimately be applied to optoelectronics and solar energy.
There’s not a direct step where somebody’s going to read this and make a new, more efficient device. But this fundamental knowledge of processes and properties is the foundation on which new technologies are built.
Bruce Weisman, Professor of Chemistry, Rice University
This study was supported by the National Science Foundation and the Welch Foundation.
Journal Reference
Lin, C.-W., et al. (2020) Delayed Fluorescence from Carbon Nanotubes through Singlet Oxygen-Sensitized Triplet Excitons. Journal of the American Chemical Society. doi.org/10.1021/jacs.0c10557.
Source: https://www.rice.edu/