Jun 22 2021
Diamond could merely be a phase that carbon experiences when it is exposed to a flash of heat, but that makes it far easier to realize.
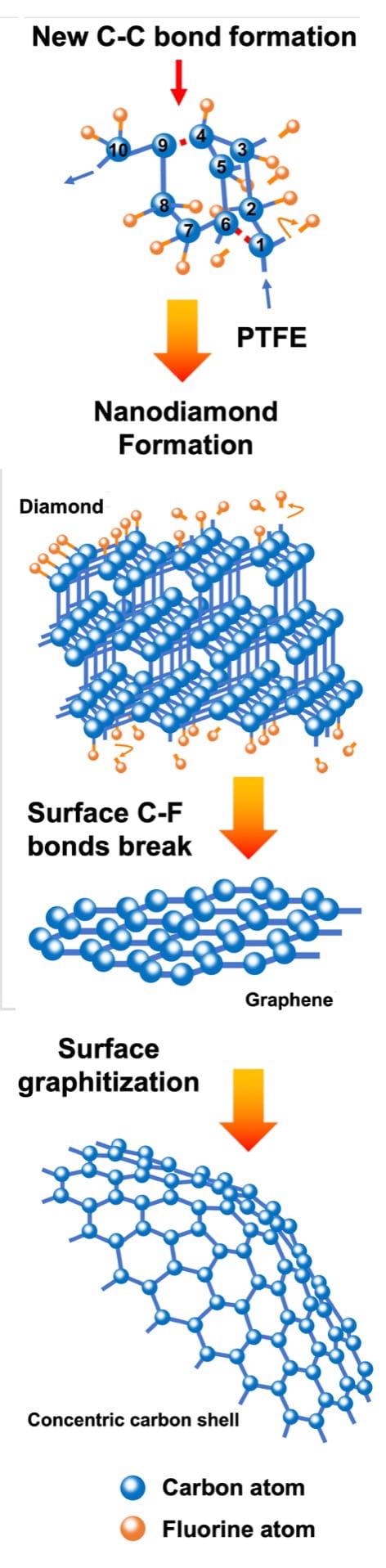 The mechanism by Rice University chemists for the phase evolution of fluorinated flash nanocarbons shows stages with longer and larger energy input. Carbon and fluorine atoms first form a diamond lattice, then graphene, and finally polyhedral concentric carbon. Image Credit: Illustration by Weiyin Chen.
The mechanism by Rice University chemists for the phase evolution of fluorinated flash nanocarbons shows stages with longer and larger energy input. Carbon and fluorine atoms first form a diamond lattice, then graphene, and finally polyhedral concentric carbon. Image Credit: Illustration by Weiyin Chen.
James Tour, a chemist from the Rice University lab, was able to “evolve” carbon via phases that contain useful nanodiamond by closely regulating the flash joule heating process developed by researchers 18 months ago. Above all, the process can be stopped at will to obtain the preferred product.
The team, guided by James Tour and Weiyin Chen, a graduate student and the lead author of the study, has revealed that introducing fluoride precursors and organic fluorine compounds to elemental carbon black converts the latter into several hard-to-get allotropes, such as fluorinated concentric carbon, fluorinated turbostratic graphene and fluorinated nanodiamonds when flashed by heat. The study was published in the American Chemical Society journal ACS Nano.
In the flash process, which was launched in 2020, a powerful jolt of electricity can convert carbon from almost any source into pristine layers of turbostratic graphene within a second. (The term “Turbostratic” means that the layers are not powerfully bound to one another, rendering them simpler to isolate in a solution.)
The new study has revealed that the products could be modified or functionalized simultaneously. The flash duration, between 10 and 500 ms, defines the final carbon allotrope. Yet, the challenge lies in the preservation of the fluorine atoms because the extreme temperature causes the volatilization of all atoms, except carbon.
To address this issue, the researchers utilized a teflon tube sealed with high-melting-point tungsten rods and graphite spacers, which can keep the reactant within, and prevent the loss of fluorine atoms under the ultrahigh temperature. Tour added that the enhanced sealed tube is very significant.
In industry, there has been a long-standing use for small diamonds in cutting tools and as electrical insulators. The fluorinated version here provides a route to modifications of these structures. And there is a large demand for graphene, while the fluorinated family is newly produced here in bulk form.
James Tour, Chemist, Rice University
Nanodiamonds are essentially microscopic crystals, or regions of crystals, that have the same carbon-atom lattice as that of macro-scale diamonds. Initially discovered in the 1960s, nanodiamonds were created under high pressure and heat from detonations.
In the past few years, scientists have identified chemical processes to make the exact same lattices. Then in 2020, a report from Boris Yakobson, a theorist from Rice University, has described how fluorine can help create nanodiamonds without high pressure, and Tour’s laboratory has demonstrated this by using pulsed lasers to convert teflon into fluorinated nanodiamond.
Nanodiamonds are quite suitable for electronics applications because they can be doped to act as wide-bandgap semiconductors — crucial components in the present study by Rice University and the Army Research Laboratory.
The novel process streamlines the doping part, both for nanodiamonds and other allotropes. The Rice lab is also investigating the use of nitrogen, phosphorous and boron as additives, added Tour.
At prolonged flash times, the team obtained nanodiamonds integrated into concentric shells of fluorinated carbon. Considerably longer exposure fully turned the diamond into shells, from the inside out.
The concentric-shelled structures have been used as lubricant additives, and this flash method might provide an inexpensive and fast route to these formations.
James Tour, Chemist, Rice University
The co-authors of the study are Rice graduate students John Tianci Li, Zhe Wang, Wala Algozeeb, Emily McHugh, Kevin Wyss, Paul Advincula, Jacob Beckham and Bo Jiang, research scientist Carter Kittrell, and alumni Duy Xuan Luong and Michael Stanford.
Tour is the T.T. and W.F. Chao Chair in Chemistry and also a professor of computer science and of materials science and nanoengineering, at Rice University.
Journal Reference:
Chen, W., et al. (2021) Ultrafast and Controllable Phase Evolution by Flash Joule Heating. ACS Nano. doi.org/10.1021/acsnano.1c03536.
Source: https://www.rice.edu/