Novel COVID-19 research on a modular vaccine platform has been posted to the Research Square / Scientific Reports preprint* server. The researchers present a peptide nanoarray scaffold vaccine that may prove useful for infection control of the severe acute respiratory coronavirus 2 (SARS-CoV-2).
Study: Peptide Nanoarray Scaffold Vaccine for SARS-COV-2 and Its Variants of Concerns. Image Credit: M-Foto/Shutterstock.com
Novel Vaccine
The transmissibility of the SARS-CoV-2 (COVID-19) virus has furthered concerns about infection control, and this has only been heightened by its mutations and emerging variants.
The variants of concern for the COVID-19 virus have enhanced its impact on populations worldwide, with public health warnings and sanctions focused on controlling the spread.
This has included national lockdowns, curfews, non-essential businesses such as retail stores being temporarily closed to prevent unnecessary gatherings, as well as mask-wearing, isolations and social distancing.
Another method to reduce the severity of the infection was through the use of vaccines.
The pandemic was established by the World Health Organization (WHO) on 11th March 2020, causing researchers and pharmaceutical companies to respond rapidly with an effective vaccine.
These vaccines involved either a viral vector or mRNA-based approach which focused on the spike protein of the SARS-CoV-2 virus. While they have been effective in decreasing the severity of the virus, with the latest variant being mild and comparable to cold-like symptoms, the effect of these vaccines may be limited for individuals with immune disorders.
The immunocompromised have become a concern for healthcare providers due to being at a greater risk of infection, as well as harboring the infection for a longer period of time than a healthier individual.
This can cause more harm to the immunocompromised and vulnerable groups as well as increase the probability of transmission to others.
Additionally, limitations of conventional vaccines that comprise whole pathogens, also include adverse side effects, such as vaccine enhanced infection, interfering neutralization antibodies, toxicity as well as certain percentages of vaccinated individuals who experience cardiovascular complications. These unwanted results can be even more catastrophic to those who are immunocompromised.
These undesirable effects from current vaccines have led innovative researchers to develop a novel vaccinology approach with a proof-of-concept comprising the use of a modular epitope vaccine against the COVID-19 virus.
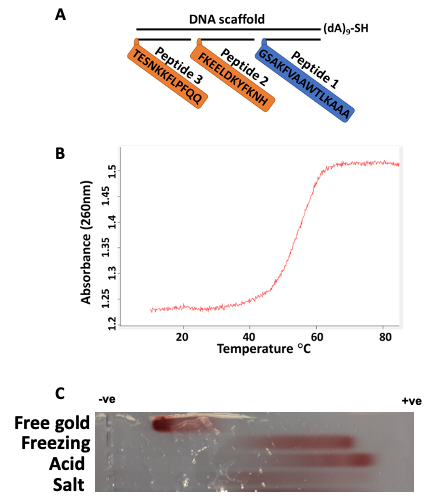
Figure 1. Schematic representation of immunogen and its validation. Three epitopes as peptides (P) 1, 2, and 3 are assembled on a DNA scaffold containing thiol for coupling with gold. According to Fig. S1, P1 is a universal T helper cell epitope PADRE, P2 is the S-protein-derived peptide (residues 1148-1159), important for cell membrane fusion, and P3 is the peptide located after RBD (residues 553-564), important for receptor recognition (A). Immunogen assembly was validated using spectrophotometric absorbance at 260 nm and the melting curve was taken at a temperature range from 10 to 85 °C (B). Immunogen-gold formulations generated by different methods characterized by agarose gel electrophoresis. From top to bottom: free gold nanoparticles, freezing-based conjugation of immunogen, acid-based conjugation, salt-based conjugation. (C). © Zagorski, K.,et al (2022)
Innovative Research
This current pre-print study has based its strategy on short peptide epitopes that produce immune responses specifically aimed at critical parts of the coronavirus, with the objective to reduce adverse effects.
Interestingly, this rational design of an immunogen consisted of two defined B-cell epitopes from the spike protein of SARS-Co-V2 and as well as a universal T-helper epitope, Pan HLA DR-binding Epitope (PADRE) that was assembled on the DNA scaffold.
The findings, which encompassed peptides constructed on a DNA scaffold, resulted in a highly effective immunogen that was able to produce a strong immune response – critical for vaccine development.
The modular vaccine development involved chemical conjugation to gold nanoparticles, this was significant as the electrophoresis data had illustrated the impact of the density of DNA coverage of the gold nanoparticle, which was a primary factor that increased the immunological efficiency of the immunogen complex.
The researchers utilized two peptides that targeted well-identified linear epitopes of the SARS-CoV-2 spike protein.
The antigenicity portion of the vaccine was included through the use of the epitope, PADRE, which was attached to the corresponding DNA probe that incorporated it into the immunogen.
Ultimately, the team used various immunogen formulations for immunizing mice to effectively evaluate the subsequent effect on the spike protein as well as the peptide-specific immune response.
The results of this innovative vaccine approach, which prioritized adverse effects, consisted of an effective immune response without the occurrence of unwanted side effects.
The serum taken from the immunized mice was found to neutralize the coronavirus as well as its variants of concern.
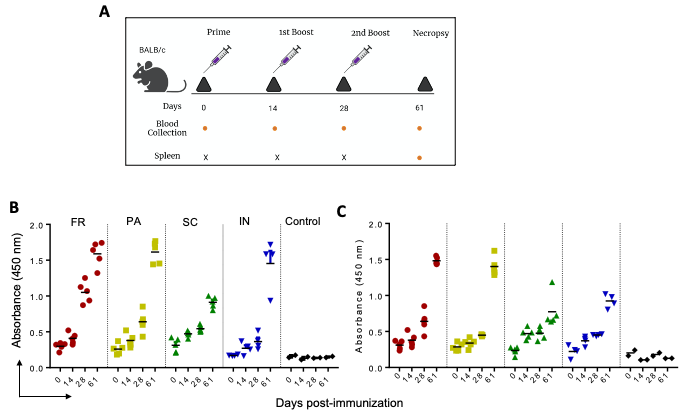
Figure 2. IgG immune response against P1 and P2 in mice immunized with GNP-conjugated vaccine candidates. BALB/c mice were immunized with GNP-conjugated vaccine candidates at different time intervals (Day 0, 14, 28, and 61), blood and spleen were collected as shown in schema (A). High protein binding 96 well plates were separately coated with P1 and P2 (100 ng/well) overnight at 4 °C. Sera prepared from different time points of immunization were diluted and incubated for 1 h at room temperature. IgG immune response against P1 (B) and P2 (C) was determined using HRP-conjugated goat anti-mouse IgG in the presence of TMB as substrate. © Zagorski, K.,et al (2022)
Translatory Implications
This is the first epitope vaccine produced through DNA hybridization-based assembly of separate epitopes made into a single molecule against the SARS-CoV-2 virus. With significantly positive results, the implication of this peptide nanoarray scaffold vaccine research consisted of providing an insight into a novel approach to vaccines as well as illuminating the critical nature of adverse effects from conventional vaccinations.
These effects can be very detrimental to patients, from being unwell for a short period of time, being unable to partake in everyday tasks due to localized pain in the vaccination area, as well as more pronounced effects, such as vomiting, muscle ache and fever.
The detrimental effects including myocarditis, cardiovascular complications and toxicity can cause irreversible damage to healthy individuals and can be mortality-inducing in the immunocompromised, that are more susceptible to infection and may not be able to recover as easily.
The translation of this well-targeted epitope vaccine, which can be highly accessible and affordable, enables protection with minimal stimulation of the immune system; this research could be revolutionary, not only for the COVID-19 pandemic and the variants of concern associated with this highly transmissible virus but also for all types of vaccines.
The aim to reduce adverse effects from vaccines would prioritize patient care and ensure more benefit than harm with its use, however, further research would be required to confirm this proof-of-concept research.
*Important Notice
Research Square / Scientific Reports publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive or treated as established information.
Reference
Zagorski, K., Pandey, K., Rajaiah, R., Olwenyi, O., Bade, A., Acharya, A., Johnston, M., Filliaux, S., Lyubchenko, Y. and Byrareddy, S., (2022) Peptide Nanoarray Scaffold Vaccine for SARS-COV-2 and Its Variants of Concerns. Research Square / Scientific Reports. Available at: https://www.researchsquare.com/article/rs-1206402/v1
Further Reading
Cucinotta D, Vanelli M. (2020) WHO Declares COVID-19 a Pandemic. Acta Biomed. 19;91(1):157-160. Available at: https://www.mattioli1885journals.com/index.php/actabiomedica/article/view/9397
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.