Proteins are present in every biological process and utilize mechanical movements to change their shape using energy from the body. Since even the tiniest structural change in a protein has a major impact on biological processes, they are referred to as biological “nanomachines.” Nanomachines that resemble proteins are being developed to implement movement in the biological environment.
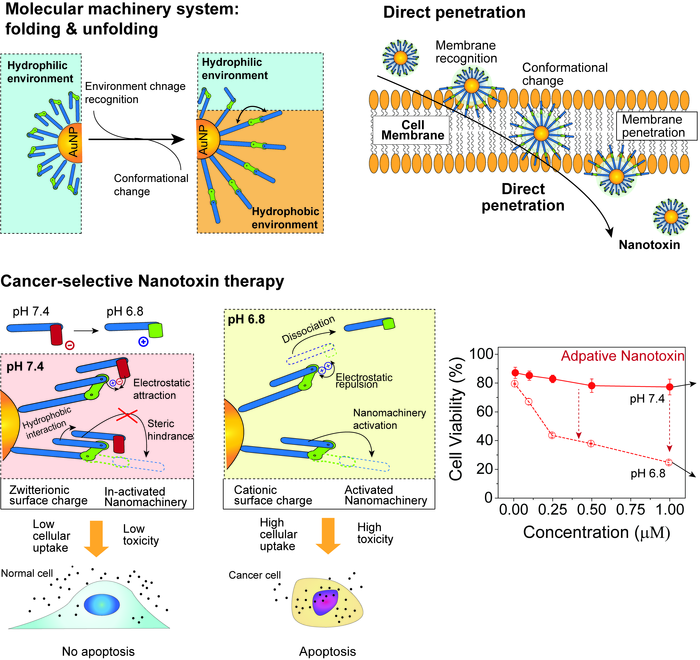 Nanomachine, developed by the KIST-UNIST joint research team, which selectively penetrates and kills cancer cells, and their mechanism of action. Image Credit: Korea Institute of Science and Technology.
Nanomachine, developed by the KIST-UNIST joint research team, which selectively penetrates and kills cancer cells, and their mechanism of action. Image Credit: Korea Institute of Science and Technology.
Cells attempt to defend themselves against the effect of these nanomachines in a variety of ways. This restricts the implementation of any important mechanical movement of nanomachines for medical applications.
The research team headed by Dr. Youngdo Jeong from the Center of Advanced Biomolecular Recognition in the Korea Institute of Science and Technology (KIST, President Seok-Jin Yoon) has described the effect of a novel biochemical nanomachine that infiltrates the cell membrane and kills the cell via molecular folding and unfolding in specific cellular environments, such as cancer cells.
This was led by a collaboration with Prof. Sang Kyu Kwak’s team from the School of Energy and Chemical Engineering and Prof. Ja-Hyoung Ryu from the Department of Chemistry in the Ulsan National Institute of Science and Technology (UNIST, President Yong Hoon Lee), and Dr. Chaekyu Kim of Fusion Biotechnology, Inc.
The combined research team focused on the hierarchical structure of proteins, in which the big structure’s axis and mobile components are separated hierarchically. As a result, only some pieces can rotate around the axis. The mobile components and axis of the big structure are present on the same layer in the majority of existing nanomachines. As a result, these components move at the same time, complicating the required control of a specific part.
Synthesizing and combining 2 nm-diameter gold nanoparticles using molecules that can be folded and unfolded dependent on the surrounding environment resulted in a hierarchical nanomachine.
This nanomachine was made up of mobile organic molecules and inorganic nanoparticles that served as large axis structures, defining movement and direction in such a way that when it reached the cell membrane, it caused a mechanical folding/unfolding movement that allowed the nanomachine to directly penetrate the cell, destroy organelles and induce apoptosis.
In contrast to capsule-type nanocarriers that convey therapeutic drugs, this new technique directly destroys cancer cells by mechanical movements without the need for anticancer drugs.
A latch molecule was then threaded onto the nanomachine to regulate the mechanical action and kill cancer cells selectively. Only a low pH environment allowed the threaded latch molecule to be released. As a result, nanomachine movements were restricted in normal cells with a rather high pH (about 7.4), and they were unable to penetrate the cell.
The latch molecules were loosened in the low pH environment surrounding cancer cells (about 6.8), allowing mechanical mobility and cell entry.
The developed nanomachine was inspired by proteins that perform biological functions by changing their shape based on their environment. We propose a novel method of directly penetrating cancer cells to kill them via the mechanical movements of molecules attached to nanomachines without drugs. This could be a new alternative to overcome the side effects of existing chemotherapy.
Dr. Youngdo Jeong, Center for Advanced Biomolecular Recognition, Korea Institute of Science and Technology
Journal Reference:
Jeong, Y., et al. (2022) Stimuli-Responsive Adaptive Nanotoxin to Directly Penetrate the Cellular Membrane by Molecular Folding and Unfolding. Journal of the American Chemical Society. doi.org/10.1021/jacs.2c00084.
Source: https://www.nst.re.kr/eng/index.do