Strong demand for adaptable CO2 capture systems capable of offering efficient solutions under varying environmental circumstances is driving research interest in reducing atmospheric CO2.

Study: Experimental and theoretical study of the effect of different functionalities of graphene oxide/polymer composites on selective CO2 capture. Image Credit: Dmitry Demidovich/Shutterstock.com
A recent study published in the journal Scientific Reports investigates the integration of graphene oxide (GO) and functionalized aqueous polymer particles for flexible CO2 capture applications. The study also intends to build a CO2 capture technology that is readily scalable, affordable, and ecologically beneficial.
CO2 Capture Technologies: Why Are They Important?
Substantial and continual amplification of greenhouse gases in the environment has become one of the most basic and persistent concerns worldwide since fossil energy sources are still inexpensive and emerging nations are in the midst of economic expansion. Among other gases, carbon dioxide (CO2) is a significant participant in the global-warming scenario.
Although the worldwide CO2 capture capability had reached 40 million tons by 2020, this is inconsequential since gigatons of CO2 must be caught each year to influence global warming majorly.
Enhancing CO2 capture and reducing global warming are some of the most difficult environmental concerns facing the 21st-century society since green energy solutions are still far from replacing fossil fuels.
As a result, creating a reliable, sensitive, and low-cost CO2 capture system is critical for our ecosystem's sustainability. Chemical or physical adsorption, membrane filtration, and enzymatic transformation have all surfaced as possible CO2 capture methods.
Graphene-Based Adsorbents for CO2 Capture
Many adsorbents for CO2 capture have been suggested, including porous polymeric materials, metal-organic complexes, alumina-based compounds, and metal oxides. However, most of them have limited adsorption capabilities, poor thermal stability, and poor selectivity compared to other gases.
Carbon-based adsorbents are emerging as a potential option to address most of the disadvantages associated with CO2 capture. This is because carbon-based adsorbents have great adsorption capabilities and comparatively low energy needs. Furthermore, characteristics such as wide surface area, durability in cycle operations, and quick adsorption kinetics support them as promising CO2 capture adsorbents.
Among carbon-based adsorbents, graphene and its compounds have been widely explored for commercial CO2 capture applications because of their minimal manufacturing costs.
The introduction of conducting polymers to graphene sheets and surface modification enhances their physicochemical qualities and facilitates handling, making these composite particles more robust and stable in cyclic processes such as CO2 capture.
Highlights of the Current Study
Computational chemistry has shown to be an effective technique in materials engineering. Density functional theory (DFT) computations, in particular, can offer a profound perspective into the dynamics between CO2 and various molecules, providing important information about possible novel adsorbents for CO2 capture technologies.
Although graphene oxide (GO)/polymer composites are extremely intriguing materials for commercial applications, there has been little research into the implications of various functionalities of GO/polymer composites on selective CO2 capture, both experimentally and theoretically. Moreover, there is no evidence in the literature on the theoretical calculation of CO2 adsorption binding energies.
Keeping the above considerations in mind, the researchers in this work used quantum chemistry to calculate the binding energy of CO2 with a variety of GO/polymer composites. Density functional theory (DFT) simulations were used to investigate the interactions between CO2 and several functional monomers, including 2-aminoethyl methacrylate hydrochloride (AEMH), hydroxyethyl methacrylate (HEMA), glycidyl methacrylate (GMA), and sodium 4-vinylbenzenesulfonate (NaSS).
The composites were specifically developed to provide a foundation for comparisons with theoretical findings. The synthetic composites were experimentally investigated to establish their suitability for CO2 capture applications.
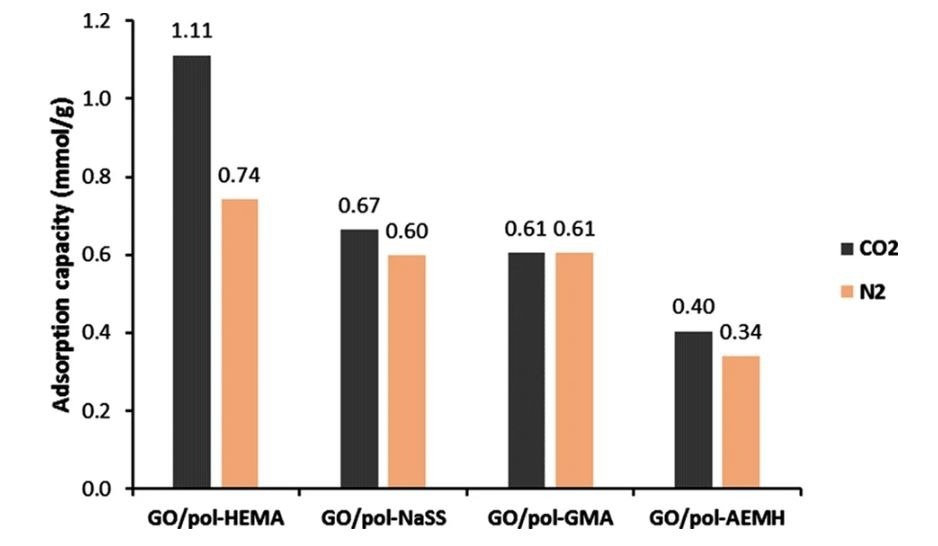
Adsorption of CO2 and N2 of the functionalized GO/polymer composites. © Stankovic, B. et al. (2022)
Important Findings of the Research
The computations revealed that the binding energy for CO2 was enhanced for the AEMH and GMA composites, whereas it was lowered for NaSS and HEMA. This surprising finding, in which the GO seems to have no cooperative impact, may be explained by a tremendously strong contact between the polymeric material and GO.
These four compounds were also experimentally manufactured, and their CO2 capture performance was investigated. The theoretical analysis accurately anticipated the CO2 capture patterns seen experimentally.
Furthermore, the disparity between theoretical and actual findings in the instance of HEMA functionalized composite showed the significance of the morphological effect. In conclusion, this study demonstrates that DFT may be a valuable method for evaluating functional polymers for developing and synthesizing novel CO2 capture materials.
Reference
Stankovic, B. et al. (2022). Experimental and theoretical study of the effect of different functionalities of graphene oxide/polymer composites on selective CO2 capture. Scientific Reports. Available at: https://www.nature.com/articles/s41598-022-20189-5
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.