Of all the known substances, hydrogen is said to possess the highest energy density of 120 MJ/kg, which is around three times more than gasoline or diesel, meaning it could have a pivotal role to play in sustainable energy platforms.
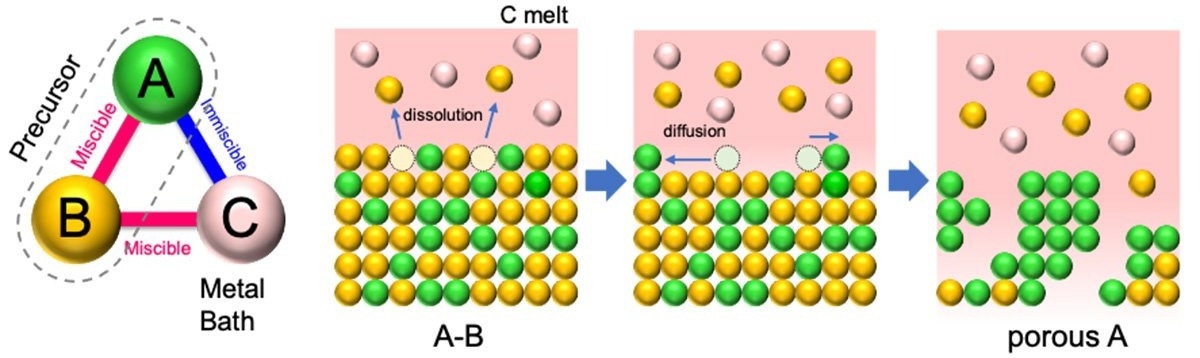 The principle and self-organizing process of liquid metal dealloying. In the precursor alloy (AB), the pore-forming metal (A) and sacrificial component (B) should have positive and negative enthalpy when mixing with the melt bath (C), respectively. With component B selectively dissolving into C melt, the remaining component A self-organizes into a porous structure. Image Credit: ©Takeshi Wada and Ruirui Song.
The principle and self-organizing process of liquid metal dealloying. In the precursor alloy (AB), the pore-forming metal (A) and sacrificial component (B) should have positive and negative enthalpy when mixing with the melt bath (C), respectively. With component B selectively dissolving into C melt, the remaining component A self-organizes into a porous structure. Image Credit: ©Takeshi Wada and Ruirui Song.
However, for the efficient creation of hydrogen by simple water splitting, high-performing catalysts are required.
Currently, a collaborative team from Tohoku University and Johns Hopkins University has created nanoporous molybdenum-based intermetallic compounds that could enhance the production of hydrogen.
Nanoscale intermetallic compounds made from non-precious transition metals possess the potential to be economical and strong catalysts for hydrogen production. Nevertheless, the creation of monolithic intermetallic compounds with sufficient active sites and adequate electrocatalytic activity continues to be a challenge for researchers.
Our research has played a crucial part in addressing that problem. Focusing on design and engineering, we harnessed an advanced dealloying technique for constructing the intermetallic compounds' architecture.
Professor Hidemi Kato, Study Co-Author, Institute for Materials Research, Tohoku University
Liquid metal dealloying is a processing method that uses the difference in the miscibility of alloy components in a molten metal bath to corrode designated component(s) while preserving the others. It facilitates self-organizing into a three-dimensional (3D) porous structure.
Moreover, it allows the pore size to be manipulated at the nanoscale for both μ-Fe7Mo6 and μ-Co7Mo6, which are mostly at the micrometer scale for the other metals/alloys when coarsening occurs at equivalent temperatures.
The collaborative team then examined the electrocatalytic performance of the novel nanoporous intermetallic compounds. It demonstrated potential for application as a commercial HER catalyst for high-current applications.
The findings from their research have been reported in the September 2nd, 2022, issue of Nature Communications.
Besides Kato, the team comprised Dr. Ruirui Song, also from the Institute for Materials Research at Tohoku University, Assistant Professor Jiuhui Han from the Frontier Research Institute for Interdisciplinary Sciences (FRIS) at Tohoku University, and Professor Mingwei Chen from Johns Hopkins University.
Going forward, the team anticipates using liquid metal dealloying to create more monolithic nanoporous intermetallic compounds by investigating the central mechanisms underlying basic intermetallic phases.
Journal Reference:
Song, R., et al. (2022) Ultrafine nanoporous intermetallic catalysts by high-temperature liquid metal dealloying for electrochemical hydrogen production. Nature Communications. doi.org/10.1038/s41467-022-32768-1.
Source: http://www.tohoku.ac.jp/en