Dec 1 2022Reviewed by Alex Smith
Materials made of nanodiamonds could be used as inexpensive photocatalysts. But up until now, high-energy UV light was needed for these carbon nanoparticles to become active. The DIACAT consortium has created and examined various nanodiamond materials.
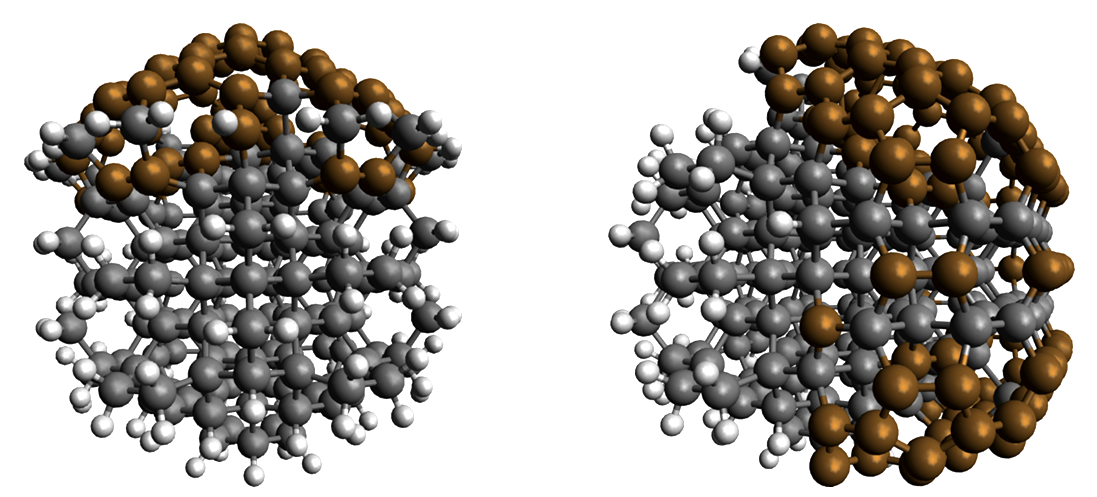
The illustration shows two variants of nanodiamond materials with different surfaces: C230H106 on the left, C286H68 on the right. Sp3 C atoms (diamond) black, sp3× C atoms (fullerene-like) brown, H atoms: Light grey. When the surface is partially covered by hydrogen atoms, nanodiamonds can absorb light in the visible range and emit electrons into solution. Image Credit: T. Kirschbaum / HZB
The research demonstrates that even the weaker energy of blue sunlight can excite nanoparticles if there are enough hydrogen atoms on their surface. Future nanodiamond-based photocatalysts could be able to use sunlight to transform CO2 or N2 into hydrocarbons or ammonia.
The potential of nanocrystalline materials as catalysts is enormous. Very large surfaces, in relation to their volume, are provided by inexpensive carbon nanoparticles. However, electrons from the catalyst must enter solvation to catalyze the acceleration of chemical reactions in an aqueous medium, and this requires high-energy UV light for excitation in pure diamond materials.
On the other hand, new molecular states that absorb visible light are permitted on the surfaces of nanodiamonds due to the nanoparticles’ incredibly small sizes.
Different Surfaces
An HZB team has now studied various nanodiamond material variations during light excitation and analyzed the processes with incredibly high time resolution as part of the DIACAT project.
 Image Credit: Kateryna Kon/Shutterstock.com
Image Credit: Kateryna Kon/Shutterstock.com
The team of Dr Jean-Charles Arnault, CEA, France, and Prof. Anke Krueger, who is currently at the University of Stuttgart, created nanodiamond samples with various surface chemistries. The surfaces of the nanoparticles varied in the quantity of oxygen or hydrogen atoms they had.
Hydrogen Helps-and Fullerene-Like Carbon Too
The hydrogen on the surfaces makes electron emission much easier. Among the many variants, we discovered that a certain combination of hydrogen as well as fullerene-like carbon on the surfaces of the nanoparticles is ideal.
Dr Tristan Petit, Leader, Young Investigator Group Nanoscale Solid-Liquid Interfaces, Helmholtz-Zentrum Berlin
Ultrafast Laser Excitations
Aqueous nanodiamond dispersions with various surface terminations, such as hydrogen, -OH, or -COOH, were investigated in the Laserlab at HZB after being excited by ultrafast laser pulses.
We were able to experimentally measure exactly how the absorption profile behaves with different excitation wavelengths in the UV range at 225 nm and with blue light in the visible range at 400 nm.
Dr Christoph Merschjann, Research Associate, Helmholtz-Zentrum Berlin
Picoseconds After the Excitation
Dr Merschjann added, “We wanted to find out what happens in the first crucial picoseconds after excitation with light, because that is the time when an electron leaves the surface and goes into the water.”
Density functional theory modeling was provided by the theory group under the direction of Dr Annika Bande to interpret the spectra. The results demonstrated that, contrary to expectations, visible light was also effective in bringing electrons into solution in samples with fullerene-like carbon on their surfaces.
Blue Light Can Work
Dr Petit stated, “In this work we show—to the best of our knowledge for the first time—that the emission of solvated electrons from nanodiamonds in water is possible with visible light!”
This marks a significant development in the development of nanodiamond materials as photocatalysts. In the future, these cheap and metal-free materials might be essential for converting N2 into ammonia or even processing CO2 into valuable hydrocarbons with sunlight.
Under Grant Agreement No. 665085, the European Union’s Horizon 2020 Research and Innovation Program has provided funding to DIACAT.
Journal Reference:
Buchner, F., et al. (2022) Early dynamics of the emission of solvated electrons from nanodiamonds in water. Nanoscale. doi:10.1039/D2NR03919B.
Source: https://www.helmholtz.de/en/