Research on new, effective, renewable, and clean energy sources is a top priority as the world's energy demand escalates. Less than 40% of the world’s energy needs are now met by renewable energy sources like the sun, wind, tidal, and geothermal energy. The world needs to improve on existing renewable energy solutions or find effective solutions to reduce the reliance on fossil fuels.
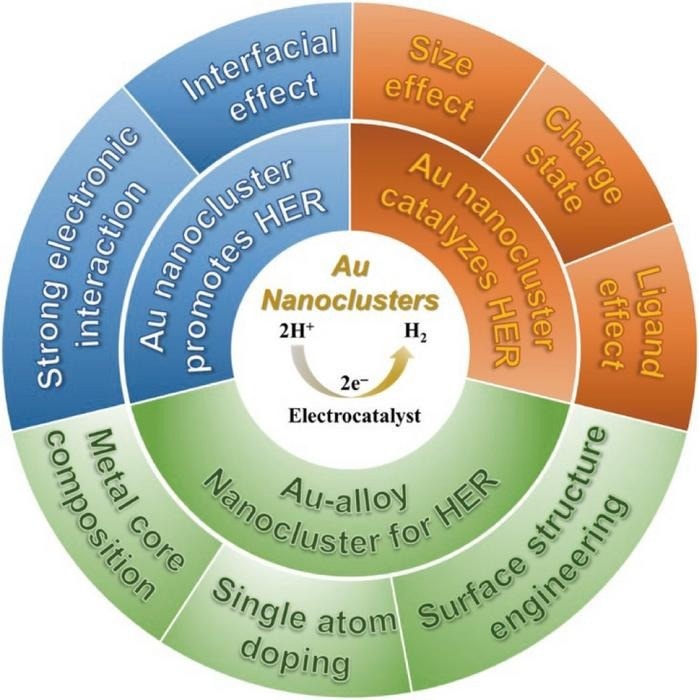 This graphic shows the different properties of gold nanoclusters that make it such a beneficial addition to HER catalysis. Image Credit: Polyoxometalates, Tsinghua University Press
This graphic shows the different properties of gold nanoclusters that make it such a beneficial addition to HER catalysis. Image Credit: Polyoxometalates, Tsinghua University Press
Hydrogen is a viable alternative, but it is currently generated by inefficient steam reforming and produces CO2 as a byproduct. Electrochemical water splitting, commonly known as water electrolysis, is one potentially effective way to manufacture hydrogen.
Water splitting necessitates a process known as the hydrogen evolution reaction (HER), however, the nanocatalysts engaged in this HER lack consistent size, composition, structure, and chemical coordination environment to increase efficiency and boost reaction mechanistic knowledge. The solution might be found in atomically accurate gold nanoclusters.
The researchers outline previous work that explores how gold nanoclusters could boost catalytic activity and promote HER in a literature review published in Polyoxometalates on August 19th, 2023.
It is extremely difficult to achieve a model catalyst with absolute uniform size, definite geometric configuration, and a well-defined local chemical environment at the anatomical level to establish the unambiguous atomical-level structure-performance relationship. Atomically precise gold nanoclusters can potentially resolve those issues. Specifically, gold nanoclusters have demonstrated extraordinary catalytic properties in various organic reactions and electrocatalytic reactions.
Zhenghua Tang, Researcher, New Energy Research Institute, South China University of Technology
For various reasons, gold nanocluster is ideally suited to be a HER catalyst. Gold nanocluster, unlike other nanocatalysts, has a perfect nanostructure. Because of this perfect structure, all gold nanoclusters are the same size, composition, shape, and chemical environment. It is also useful in determining the active sites of HER catalysis. Gold nanoclusters’ high chemical reactivities enable metal core tailoring as well as surface ligand engineering.
Metal core tailoring is the addition of another metal to a gold nanocluster, resulting in a gold-alloy cluster. The use of another metal can provide fresh catalytic properties while also lowering the cost. Surface ligand engineering is the process of fine-tuning the surface chemical environment to expose more active sites or modify the shape of the nanocluster.
Finally, gold nanoclusters offer other structural advantages, such as ultrasmall size, which satisfies the notion of “small is precious” in the catalysis sector; morphology that can be tweaked and controlled; and strong stability with the entire structure retained in diverse reactions under mild circumstances.
Tang added, “The cases presented in this review clearly show that exceptional HER catalytic properties are often displayed because of the distinct advantages of gold nanoclusters compared to gold nanoparticles. However, challenges are certainly present in employing gold nanoclusters for HER catalysis.”
Finding a solution to the amount of gold necessary to scale the usage of these catalysts, concerns with how the nanocatalysts work in harsh settings and faulty theoretical modeling are some of the main obstacles connected with gold nanoclusters.
Looking ahead, researchers are considering the next stages in nanocatalyst research. Testing the applicability of the gold cluster-based composite for various reactions combined with HER and increasing the electrical conductivity of the cluster-based composite catalyst are suggested routes.
Tang concluded, “Owing to the rapid development of synthetic techniques and catalysis science, we anticipate more research efforts will be dedicated to using atomically precise metal nanoclusters as model catalysts for various electrocatalytic reactions and beyond.”
This study was also supported by Xin Zhu, Leyi Chen, and Yonggang Liu of the New Energy Institute at the South China University of Technology in Guangzhou, China.
The study was funded by the Guangzhou Basic Research and Applied Basic Research Foundation and the Guangdong Natural Science Funds.
Journal Reference:
Zhu, X., et al. (2023) Atomically precise Au nanoclusters for electrochemical hydrogen evolution catalysis: Progress and perspectives. Polyoxometalates. doi:10.26599/POM.2023.9140031
Source: http://www.tup.tsinghua.edu.cn/en/index.html