The Innovation Center of NanoMedicine, in collaboration with The University of Tokyo, has revealed that a team under the leadership of Prof. Takanori Ichiki, who serves as the Research Director of iCONM, has introduced a novel method for assessing the shape anisotropy of nanoparticles.
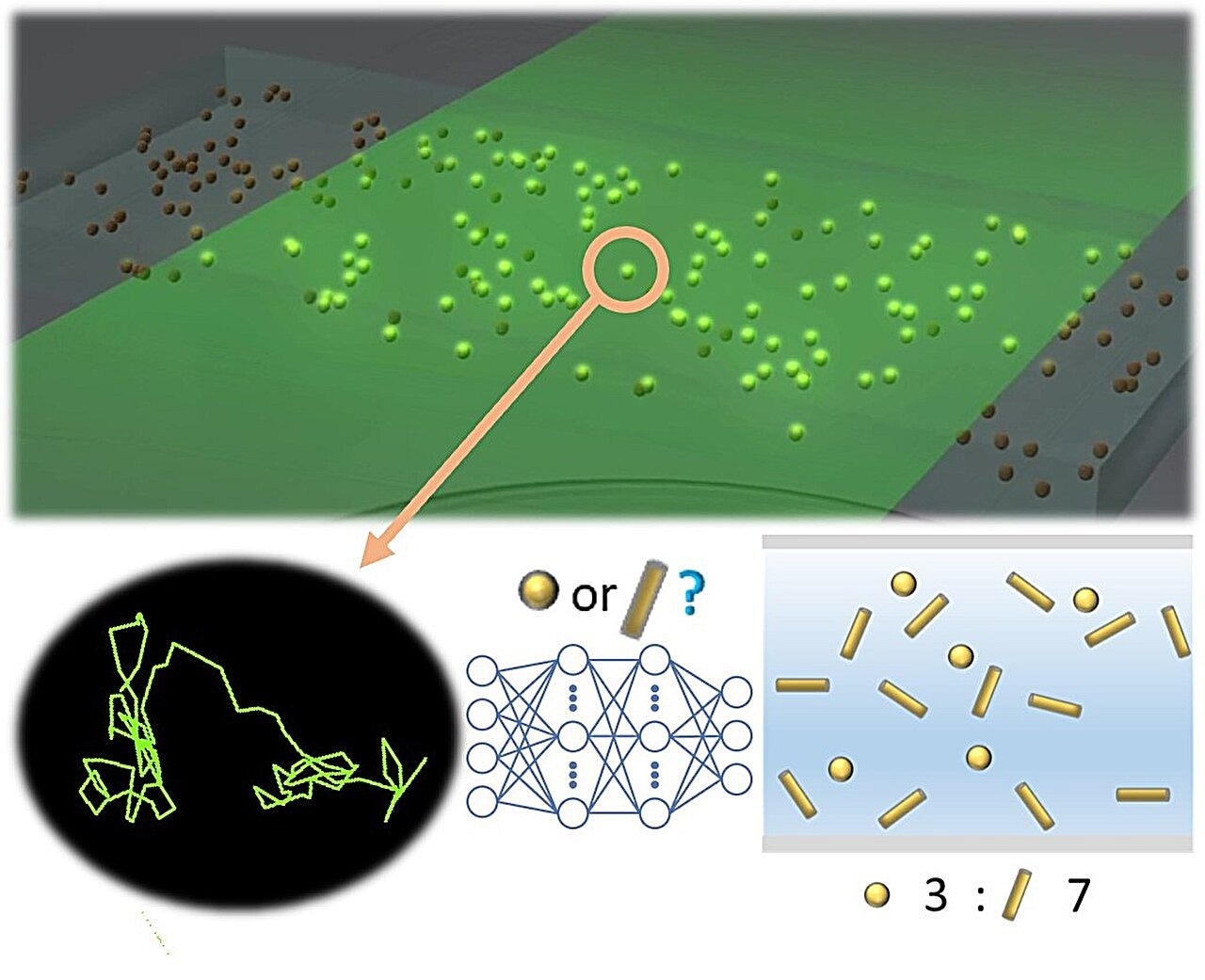 Scheme of shape prediction of nanoparticles. Image Credit: Department of Materials Engineering, Graduate School of Engineering, The University of Tokyo
Scheme of shape prediction of nanoparticles. Image Credit: Department of Materials Engineering, Graduate School of Engineering, The University of Tokyo
This method addresses longstanding challenges in nanoparticle evaluation that trace back to Einstein's era.
The study was published in APL Machine Learning.
In an age where novel medical treatments and diagnostic technologies involving extracellular vesicles and artificial nanoparticles are gaining prominence, nanoparticles have emerged as valuable materials in the domains of medicine, pharmaceuticals, and industry.
From a materials standpoint, it is imperative to assess the characteristics and aggregation state of individual nanoparticles, ensuring quality control. There is an anticipation of continued advancements in nanoparticle evaluation technology that bolster safety and reliability in their application.
One method to assess nanoparticles in a liquid medium is by analyzing their Brownian motion trajectories. This method, known as NTA (Nanoparticle Tracking Analysis), determines the particle's diameter using a theoretical formula formulated by Einstein more than a century ago.
While it serves as a straightforward means to measure individual particles ranging from micro to nano sizes, a persistent challenge has been its inability to assess the shape of nanoparticles.
The trajectory of Brownian motion does provide insights into the influence of particle shape, but accurately measuring extremely rapid motion has proven to be challenging. Moreover, when dealing with non-spherical particles, conventional analysis methods tend to be less accurate because they make the unconditional assumption that the particle is spherical, employing the Stokes-Einstein equation for analysis.
Nonetheless, by utilizing deep learning, which excels at uncovering concealed correlations within extensive datasets, it becomes feasible to identify variations stemming from shape differences. This holds even when measurement data is averaged or contains indistinguishable errors.
Prof. Takanori Ichiki's research team developed a deep learning model capable of recognizing shapes from the recorded Brownian motion trajectory data without any alterations to the experimental procedure.
To account for both the temporal changes in the data and the correlation with the surrounding environment, they incorporated a 1-dimensional CNN (Convolutional Neural Network) model, known for extracting local features through convolution, and a bidirectional LSTM (Long Short-Term Memory) model, capable of accumulating temporal dynamics.
By employing trajectory analysis with the integrated model, they managed to attain a classification accuracy of around 80% on an individual particle basis for two types of gold nanoparticles. These nanoparticles are of roughly the same size but exhibit distinct shapes, a differentiation that conventional NTA alone cannot achieve.
This remarkable precision signifies that, for the first time, deep learning analysis has achieved a practical level of accuracy in classifying the shapes of individual nanoparticles in a liquid. In addition, the paper introduces a calibration curve to determine the mixing ratio in a solution containing two types of nanoparticles (spherical and rod-shaped). Considering the diverse range of nanoparticle shapes found worldwide, this method is believed to be effective in shape detection.
Traditional NTA approaches do not allow for the direct observation of particle shape, and the information acquired is rather restricted. Although the trajectory of Brownian motion, represented by time-series coordinate data measured by the NTA device, holds clues about nanoparticle shape, the rapid relaxation time has made it challenging to effectively discern the anisotropy of particle shapes.
Moreover, in conventional analysis techniques, the accuracy is compromised when dealing with non-spherical particles because the shape factor is not considered; instead, spherical assumptions are made, and the Stokes-Einstein equation is used for analysis.
The researchers sought to devise a novel approach accessible to anyone and successfully addressed a longstanding challenge in Brownian motion analysis. They accomplished this by incorporating deep learning, renowned for uncovering concealed correlations within extensive datasets, into data analysis, all while maintaining the simplicity of the experimental methods.
In this research, they endeavored to identify the shapes of two particle types. However, given the diversity of nanoparticle shapes available commercially, they believe this method can be practically applied, such as in the identification of foreign substances within homogeneous systems.
The broader application of NTA is expected to extend beyond research and find utility in various industrial sectors, facilitating the evaluation of properties, agglomeration status, and uniformity of non-spherical nanoparticles, all while enhancing quality control measures.
This method is particularly anticipated to offer a solution for assessing the characteristics of diverse biological nanoparticles, including extracellular vesicles, within an environment resembling that of living organisms.
Moreover, it holds the potential to serve as an innovative approach for fundamental research concerning the Brownian motion of non-spherical particles in liquid.
Reference:
Fukuda, H., et al. (2023). Analysis of Brownian motion trajectories of non-spherical nanoparticles using deep learning. APL Machine Learning. doi.org/10.1063/5.0160979.
Source: https://iconm.kawasaki-net.ne.jp/en/index.html