Xin-Wen Zhou, from the College of Materials and Chemical Engineering at China Three Gorges University, spearheaded new research that explores a straightforward method to synthesize a range of PdFe/Cu catalysts through a step-by-step reduction process involving surface reconstruction.
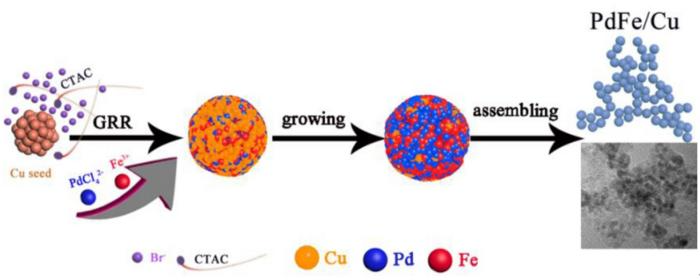 In an alkaline environment, PdCl₄²⁻ undergoes a galvanic replacement reaction (GRR) with Cu or is directly reduced by ethylene glycol (EG) to form metallic Pd. Concurrently, Fe³⁺ reacts with Cu, leading to the rapid generation of Cu²⁺ and Fe²⁺. Art by Zhou’s group. Image Credit: Beijing Zhongke Journal Publishing Co. Ltd.
In an alkaline environment, PdCl₄²⁻ undergoes a galvanic replacement reaction (GRR) with Cu or is directly reduced by ethylene glycol (EG) to form metallic Pd. Concurrently, Fe³⁺ reacts with Cu, leading to the rapid generation of Cu²⁺ and Fe²⁺. Art by Zhou’s group. Image Credit: Beijing Zhongke Journal Publishing Co. Ltd.
The ethanol oxidation reaction (EOR) serves as a crucial anode reaction in direct ethanol fuel cells (DEFCs), traditionally relying on the noble metal platinum (Pt) as its ideal anode catalyst.
However, the high cost, limited applicability, and insufficient resistance to CO poisoning hinder the widespread adoption of this approach. Recent research indicates that synthesizing new catalysts by combining precious metals with non-noble metals and non-metals effectively reduces costs and enhances catalyst performance.
Morphology control, surface engineering, improved carrier effects, and even the adoption of a single-atomic strategy show promise in significantly boosting the utilization of precious metals.
Studies increasingly demonstrate that the electrooxidation of ethanol in alkaline media not only exhibits faster kinetics but also boasts high activity and long-term stability. In the alkaline system, the use of the more cost-effective Pd (compared to Pt) as the active material for catalytic ethanol oxidation has been explored.
Research suggests that the formation of noble metal-transition metal alloy catalysts (for example, PdFe, PdNi, PdAu, PdCo, PdAg, PdCu, and AlNiCuPtPdCo) or noble metal/nonmetal composite materials not only reduces the Pd content in the catalyst but also plays a pivotal role in enhancing the electrooxidation performance of ethanol.
In recent years, advancements in anionic solid electrolyte membranes have significantly propelled research on direct alkaline ethanol fuel cells (DAEFCs), with commercial applications on the horizon.
The increased kinetics of ethanol reaction in an alkaline solution can be attributed to the pH-induced negative shift in the working potential of 59 mV/pH, altering the local electric double layer structure and electric field distribution at the electrode/electrolyte interface, thereby enhancing electrocatalytic activity.
In an alkaline environment, the stability of active transition metals (for example, Fe, Ni, Co, Cu) can be enhanced, particularly by forming a multi-metal Pd-based catalyst. Introducing Cu2+ ions during precursor stages transforms spherical PtFe nanoparticles into PtFeCu nanochains, showcasing enhanced methanol oxidation properties.
PdFe-based catalysts, noted for their high electrical conductivity, exceptional catalytic activity, and durability, are frequently employed as electrocatalysts for the oxygen reduction reaction (ORR) or anode oxidation reaction. The synergy between the core and shell introduces stress-strain effects, altering the surface's electronic structure and influencing oxygen adsorption and reduction.
The phase transformation of face-center cubic (fcc) to face-center tetragonal (fct) in PdFe@Pd increases the catalyst’s active site, thereby enhancing ORR catalytic performance.
The assembled nano PdFe alloy film serves as a highly efficient electrocatalyst for hydrogen evolution in both acidic and alkaline solutions due to changes in the valence electron structure of Pd and an increase in the electrochemical active area (ECSA).
The galvanic replacement reaction (GRR), an in-situ sacrificial template method, is employed for hollow nanocatalyst preparation through a potential displacement method.
The stepwise co-reduction method is utilized in this study to design a PdFe/Cu nanocatalyst with a reduced particle size. The GRR process induces a strong reconstituted effect, oxidizing the surface of the Cu seed crystal and resulting in the formation of superfine PdFe/Cu nanoparticles.
The synergy and strain-stress effects among Pd, Fe, and Cu, induced by this structural change, alter the electronic structure of the nanoparticles, proving advantageous for ethanol molecule adsorption and subsequent oxidation reactions.
Comparative testing with three control catalysts (CuPdFe, PdFe, and CuPdFe/Cu) under optimized conditions reveals the catalyst with the optimal electrooxidation performance of ethanol in the alkaline system.
Journal Reference:
Chen, D., et al. (2023) Highly active and durable PdFe/Cu nanocatalysts prepared by liquid phase synthesis for ethanol electrooxidation reaction. Advanced Sensor and Energy Materials. doi.org/10.1016/j.asems.2023.100075.
Source: https://www.zhongkeqikan.com/