Although ammonia (NH3) is seen to be a viable carbon-free energy source, experts throughout the world are still puzzled by its energy-intensive manufacturing method. To produce ammonia from nitrate (NO3-), a research team led by the City University of Hong Kong (CityU) recently engineered a bimetallic alloy as an ultrathin nanocatalyst that can provide significantly enhanced electrochemical performance. This offers great potential for the future production of carbon-neutral fuel.
 Schematic illustration of the synthesis of RuFe nanoflowers for electrochemical nitrate reduction reaction (NO3RR). Image Credit: Proceedings of the National Academy of Sciences (2023). DOI: 10.1073/pnas.2306461120
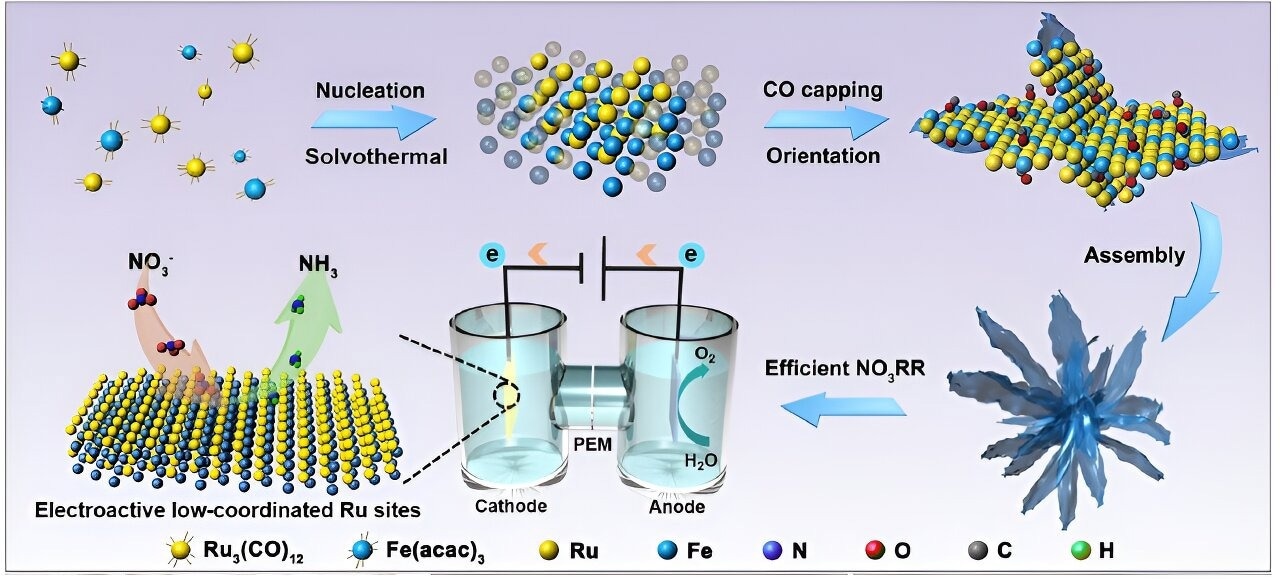
Schematic illustration of the synthesis of RuFe nanoflowers for electrochemical nitrate reduction reaction (NO3RR). Image Credit: Proceedings of the National Academy of Sciences (2023). DOI: 10.1073/pnas.2306461120
The results, titled “Atomic coordination environment engineering of bimetallic alloy nanostructures for efficient ammonia electrosynthesis from nitrate,” were published in the Proceedings of the National Academy of Sciences (PNAS) journal.
Since ammonia is easier to liquefy and transport than hydrogen, and can serve as a source of hydrogen for fuel cells, this often-used fertilizer ingredient has garnered a lot of interest lately. Upcycling nitrate (NO3-) from wastewater contaminated by ammonium fertilizers has become a viable alternative for creating useful ammonia and enhancing the sustainability of agriculture due to its high demand.
As of right now, the electrochemical nitrate reduction reaction (NO3RR) is thought to be a viable method for producing ammonia. Metal-based electrocatalysts mostly consist of deoxygenation and hydrogenation stages (NO3- + 9H+ + 8e- ➙ NH3 + 3H2O).
However, the undesired by-products and the competing hydrogen evolution reaction (HER) during NO3RR apparently hinders the yield rate of ammonia production.
Zhanxi Fan, Study Lead and Professor, Department of Chemistry, City University of Hong Kong
Unlike prior studies that altered the size or dimensions of the electrocatalysts, Professor Fan’s group concentrated on enhancing the active sites, which are the points on the electrocatalyst surface where substrate molecules attach and catalysis occurs.
Fan added, “Ruthenium (Ru) is an emerging material as an electrocatalyst for NO3RR, but it also has the problem of favoring HER, which leads to its active sites being highly occupied by undesired active hydrogen, leaving insufficient area for nitrate reduction into ammonia.”
Iron (Fe) was added by the researchers to modify the atomic coordination environment of the active sites to get around the difficulties. The electronic structures and surface characteristics of Ru, and therefore their catalytic activity for ammonia production, are adjusted by modifying the coordination environment of the Ru sites.
The group created RuFe nanoflowers, ultrathin nanosheets arranged into a flower-like shape, using a one-pot synthesis method to further improve the electrocatalyst’s efficiency.
In addition to suppressing the competitive HER and lowering the energy barriers for NO3RR, the complementary orbitals in this unique bimetallic alloy-made catalyst achieve strong valence states and a highly stable electronic structure.
In addition, the RuFe nanoflowers’ electrochemically active surface sites measured 267.5 cm2, which is far bigger than the Ru-nanosheets’ 105 cm2 required for the reactions to occur.
The electrochemical performance of RuFe nanoflowers was remarkably improved, as evidenced by their exceptional faradaic efficiency (FE) of 92.9% and yield rate of 38.68 mg h−1 mgcat−1 for ammonia production at −0.30 and −0.65 V. This yield rate is nearly 6.9 times higher than that of sole Ru-nanosheets.
Fan concluded, “This research indicates great potential for RuFe nanoflowers in next-generation electrochemical energy systems. We believe this work can stimulate follow-up studies on modulating the atomic coordination environment of active sites in metal-based catalysts for ammonia production, further promoting a sustainable nitrogen cycle to achieve carbon-free energy in the future.”
Journal Reference:
Wang, Y, et. al. (2023) Atomic coordination environment engineering of bimetallic alloy nanostructures for efficient ammonia electrosynthesis from nitrate. Proceedings of the National Academy of Sciences. doi:10.1073/pnas.2306461120
Source: https://www.cityu.edu.hk/