Feb 22 2021
Muscle plays a crucial role in maintaining the human lives and is the largest organ accounting for 40% of body mass. Muscle tissue is known for its special potential for spontaneous regeneration.
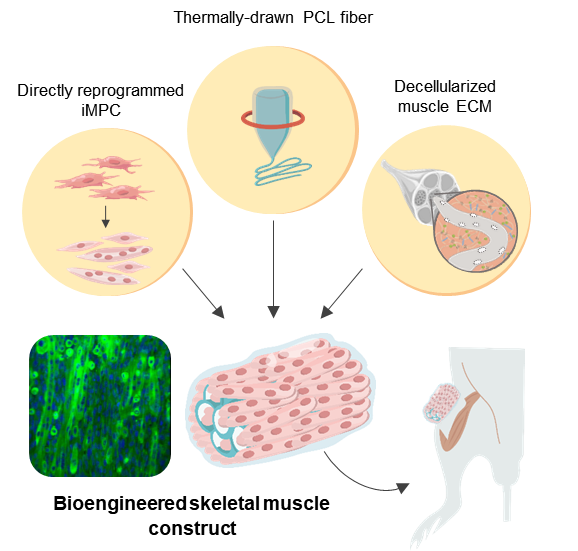 Schematic illustration of the 3D skeletal muscle-like bioengineered constructs. Image Credit: Institute for Basic Science.
Schematic illustration of the 3D skeletal muscle-like bioengineered constructs. Image Credit: Institute for Basic Science.
But in serious injuries, like those sustained in motor vehicle accidents or the resection of tumors, which leads to a volumetric muscle loss (VML), the ability of the muscle to recover is considerably reduced.
VML treatments presently involve surgical interventions with autologous muscle grafts or flaps followed by physical therapy. But surgical procedures usually lead to a decreased function of muscles and, in certain cases, lead to a complete failure of grafts. Hence, there is a need for more therapeutic options to enhance the recovery of muscle loss.
One potential method to enhance the functional capacity of the impaired muscle is to promote de novo regeneration of skeletal muscle by integrating the transplanted cells.
Different types of cells, such as satellite cells, or muscle stem cells, mesenchymal stem cells and myoblasts, have been used for treating muscle loss. But limited long-term maintenance, poor availability of cells and invasive muscle biopsies hinder clinical translation, where mature cells amounting to millions to billions may be required to offer therapeutic benefits.
Another major concern is managing the 3D microenvironment at the site of injury to make sure that the transplanted cells correctly differentiate into muscle tissues that have the required structures.
A wide range of synthetic and natural biomaterials has been utilized to improve the maturation and survival of transplanted cells and, at the same time, recruit host cells for the regeneration of muscles.
But there are still long-lasting, unresolved problems in the development of tissue scaffolds. Natural scaffolds have excellent cell binding affinity and high cell recognition but usually do not offer mechanical robustness in load-bearing tissues or large lesions that need mechanical support over the long term.
On the other hand, synthetic scaffolds offer an accurately engineered option with adjustable physical and mechanical characteristics, and also customized biochemical compositions and structures, but these scaffolds are usually impeded by substandard integration with the host tissue and the absence of cell recruitment.
Hence, to address these problems, a group of researchers from the Center for Nanomedicine within the Institute for Basic Science (IBS) based in Seoul, South Korea, Yonsei University and the Massachusetts Institute of Technology (MIT) developed a new protocol for the regeneration of artificial muscles.
By using direct cell reprogramming technology together with a natural-synthetic hybrid scaffold, the researchers were able to effectively treat VML in a mouse model.
Also called direct conversion, direct cell reprogramming is an efficient technique that offers effective cell treatment. This is because this strategy makes it possible to quickly generate patient-specific target cells with the help of autologous cells from the tissue biopsy.
Fibroblasts are essentially cells that are often found inside the connective tissues, and they play a crucial role in wound healing.
Since the fibroblasts are not actually terminally differentiated cells, they can be changed into induced myogenic progenitor cells, or iMPCs for short, using many different transcription factors. In this case, this method was used to offer iMPC for designing muscle tissues.
To give structural support to the proliferating muscle cells, the team selected polycaprolactone (PCL) as the ideal material for designing a porous scaffold because of its excellent biocompatibility.
Although salt-leaching is a technique that is extensively used for producing porous materials, it is largely restricted when it comes to creating closed porous structures. Hence, to resolve this restriction, the investigators augmented the traditional salt leaching technique using thermal drawing to create custom-made PCL fiber scaffolds.
This method enabled high throughput development of porous fibers that have controlled dimensions, porosity and stiffness that allow accurate customization of the scaffolds to the sites of injuries. But the artificial PCL fiber scaffolds do not singularly offer optimal local and biochemical mechanical cues that imitate a microenvironment specific to muscles.
The fabrication of a hybrid scaffold was finished by integrating decellularized muscle extracellular matrix (MEM) hydrogel into the PCL structure. MEM is a natural biomaterial that is now extensively used for treating VML in clinical settings.
The team, therefore, believes that hybrid scaffolds designed with MEM have an excellent potential in clinical applications.
The bioengineered muscle fiber constructs, thus obtained, exhibited mechanical stiffness that was analogous to that of muscle tissues and showed improved differentiation of muscles as well as elongated muscle alignment in vitro.
In addition, when bioengineered muscle constructs were implanted in the VML mouse model, this method promoted the regeneration of muscles with increased angiogenesis and innervation and also facilitated the functional recovery of impaired muscles.
The hybrid muscle construct might have guided the responses of exogenously added reprogrammed muscle cells and infiltrating host cell populations to enhance functional muscle regeneration by orchestrating differentiation, paracrine effect, and constructive tissue remodeling.
Study Researchers
“Further studies are required to elucidate the mechanisms of muscle regeneration by our hybrid constructs and to empower the clinical translation of cell-instructive delivery platforms” concluded Professor CHO Seung-Woo from the IBS Center for Nanomedicine and Yonsei University College of Life Science and Biotechnology, who headed the new study.
Journal Reference:
Jin. Y., et al. (2021). Functional Skeletal Muscle Regeneration with Thermally Drawn Porous Fibers and Reprogrammed Muscle Progenitors for Volumetric Muscle Injury. Advanced Materials. doi.org/10.1002/adma.202007946.
Source: https://www.ibs.re.kr/