Apr 1 2021
Amorphous solids, which also include rubber, glass and plastics, are a class of matter. But despite their routine applications in people’s day-to-day lives, amorphous solids have traditionally presented a challenge to researchers.
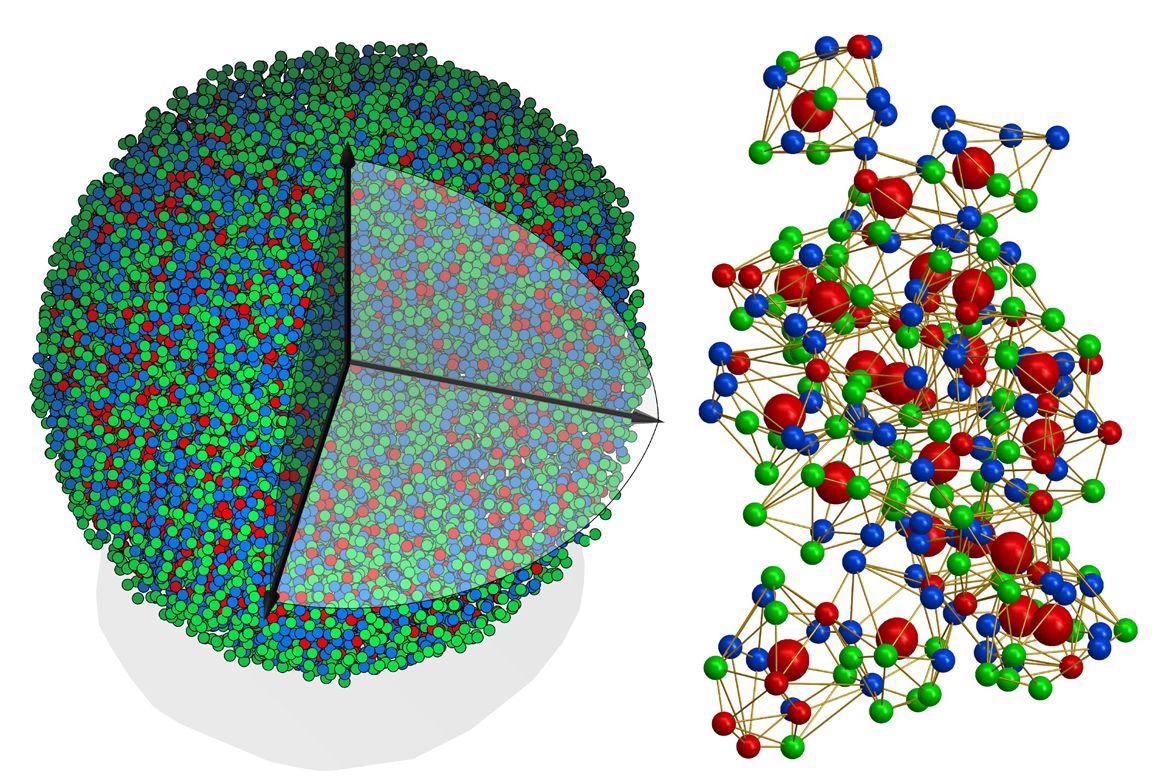 At left, an experimental 3D atomic model of a metallic glass nanoparticle, 8 nanometers in diameter. Right, the 3D atomic packing of a supercluster within the structure, with differently colored balls representing different types of atoms. Image Credit: University of California, Los Angeles.
At left, an experimental 3D atomic model of a metallic glass nanoparticle, 8 nanometers in diameter. Right, the 3D atomic packing of a supercluster within the structure, with differently colored balls representing different types of atoms. Image Credit: University of California, Los Angeles.
Since the 1910s, investigators have successfully mapped the atomic structures of crystals—the other leading group of solids—in 3D. This resulted in a wide range of developments in nanoscience, geology, materials science, biology, chemistry, physics, drug discovery, etc.
However, since amorphous solids are not organized in repetitive and rigid atomic structures like how crystals are assembled, they have challenged researchers’ potential to establish their atomic structure with the same level of accuracy, until now.
For the first time, a study headed by the University of California, Los Angeles (UCLA) has determined the 3D atomic structure of an amorphous solid—in this example, a material known as metallic glass. The study has been published in the Nature journal.
“We know so much about crystals, yet most of the matter on Earth is non-crystalline and we know so little about their atomic structure,” stated Jianwei “John” Miao, the senior author of the study and a UCLA professor of physics and astronomy. Miao is also a member of the California NanoSystems Institute at UCLA.
Ever since Miao was a graduate student, he has always wanted to observe the 3D atomic arrangement of an amorphous solid. And now, that dream has been realized following 22 years of persistent research work.
“This study just opened a new door,” Miao added.
When compared to standard crystalline metals, metallic glasses are relatively stronger and more conformable. Even today, these metallic glasses are used in many products, such as high-end golf clubs, electrical transformers, the housings for Apple laptops and other similar electronic devices.
Figuring out the atomic structure of metallic glasses can help scientists design more improved models of these materials, for an even broader range of applications.
The team employed a method called atomic electron tomography—a kind of 3D imaging invented by Miao and colleagues. In this method, electrons are beamed via a specimen, and an image is obtained on the other side.
The specimen is then rotated so that measurements can be taken from various angles and this generates data that is subsequently stitched together to create a 3D image.
We combined state-of-the-art electron microscopy with powerful algorithms and analysis techniques to study structures down to the level of single atoms. Direct knowledge of amorphous structures at this level is a game-changer for the physical sciences.
Peter Ercius, Study Co-Author and Staff Scientist, Molecular Foundry, Lawrence Berkeley National Laboratory
The experiment was carried out at the Molecular Foundry.
The team assessed a metallic glass sample that measured around 8 nm (a nanometer is one-billionth of a meter) in diameter and composed of eight different metals.
Miao and his research team then used 55 images obtained from atomic electron tomography and eventually produced a 3D map of the approximately 18,000 atoms that constituted the nanoparticle.
Since amorphous solids are highly difficult to define, the team anticipated the atoms to organize randomly. And while about 85% of the atoms were assembled chaotically, the investigators effectively identified pockets in which a part of atoms coalesced into organized superclusters.
These results prove that the arrangement of atoms is not fully random even inside an amorphous solid.
However, Miao identified one drawback in the study, which emerged from the limitation of electron microscopy itself. A few of the metal atoms were so similar in size that electron imaging found it rather difficult to differentiate between them.
For the purpose of this analysis, the team classified the metals into three categories, combining neighboring metals from the periodic table of elements, such as nickel and cobalt in the first category; silver, palladium, rhodium, and ruthenium in the second category; and platinum and iridium in the third category.
The study was mainly supported by the STROBE National Science Foundation Science and Technology Center, in which Miao serves as a deputy director. In addition, the study was partly funded by the U.S. Department of Energy.
This groundbreaking result exemplifies the power of a transdisciplinary team. It demonstrates the need for long-term support of a center to address this type of complex research project.
Charles Ying, Program Officer, National Science Foundation
Ying oversees funding for the STROBE center.
The co-first authors of the study are graduate student Yao Yang, former assistant project scientist Jihan Zhou, former postdoctoral researcher Fan Zhu, and postdoctoral researcher Yakun Yuan, all present or former members of Miao’s research team from UCLA.
Other co-authors from UCLA include graduate students Dillan Chang and Arjun Rana, former postdoctoral scholars Dennis Kim and Xuezeng Tian, assistant adjunct professor of mathematics Minh Pham, and mathematics professor Stanley Osher.
Yonggang Yao and Liangbing Hu from the University of Maryland, College Park, and Andreas Schmid and Peter Ercius from the Lawrence Berkeley National Laboratory are other co-authors of the study.
This work is a great illustration of how to address longstanding grand challenges by bringing together scientists with many different backgrounds in physics, mathematics, materials and imaging science, with strong partnerships between universities and national laboratories. This is a spectacular team.
Margaret Murnane, Director, STROBE Center
Journal Reference:
Yang, Y., et al. (2021) Determining the three-dimensional atomic structure of an amorphous solid. Nature. doi.org/10.1038/s41586-021-03354-0.
Source: https://www.ucla.edu/