The Chung Lab at USC has developed a nanoparticle that attaches to immune cells, allowing it to target lymph nodes—a vital indicator of cancer metastasis—and enhance MRI detection, potentially revolutionizing cancer screenings by improving early detection of metastasis without invasive biopsies.
 The Chung Lab has developed a nanoparticle that can hitchhike on immune cells and travel to the lymph nodes. Image Credit: Chung Lab
The Chung Lab has developed a nanoparticle that can hitchhike on immune cells and travel to the lymph nodes. Image Credit: Chung Lab
The lymph nodes are a vital early sign for the almost 20 million cancer patients worldwide who receive a diagnosis each year as to whether their disease has metastasized—that is when cancer cells start to migrate to another organ. If metastasis is discovered as soon as feasible, the patient’s prognosis will significantly improve since the required immunotherapy and chemotherapy could be provided.
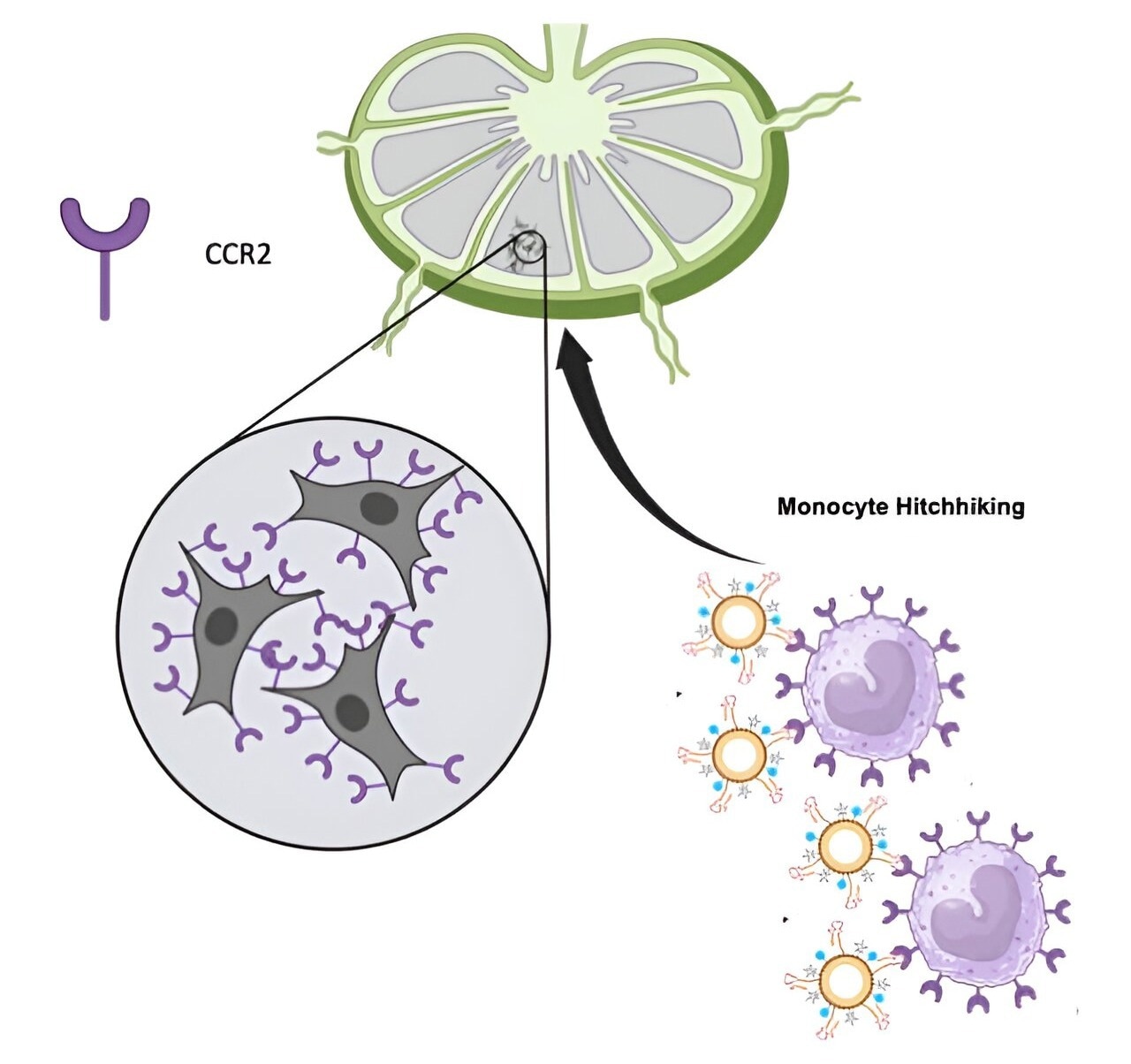
Researchers from the Alfred E. Mann Department of Biomedical Engineering at USC have created a novel nanoparticle that can “hitch a ride” on monocytes, which are immune cells. The particle’s small size allows it to attach itself directly to lymph nodes, aiding in the appearance of metastasis on MRIs when it would otherwise be difficult to see. The findings could result in enhancing lymph node MRI cancer screenings.
Dr. Karl Jacob Jr. and Karl Jacob III Early-Career Chair Eun Ji Chung, as well as Ph.D. student Noah Trac from the Chung Lab, led the study published in ACS Nano.
Although lymph nodes play a crucial role in the detection of cancer, the process of screening them by biopsy is intrusive, and painful. It can have unintended consequences such as infection, lymphedema, and thrombosis. MRI detection and other imaging technologies are non-invasive. However, they also have serious drawbacks regarding lymph node screening.
MRIs will look at the lymph node’s size, but that does not have a great connection and correlation to the fact that it is metastatic.
Eun Ji Chung, Karl Jacob III Early-Career Chair and Associate Professor of Biomedical Engineering, University of Southern California
Trac added, “The major issue with current MRI techniques is not that they don't detect the immune cells. A major issue with current contrast agents is that there is no cancer-targeting mechanism, so most lymph nodes are lit up equally, regardless of whether or not there is cancer.”
To overcome this obstacle, Chung, Trac, and their colleagues created a nanoparticle that specifically targets a receptor found on immune cells called monocytes, which are more common in disease conditions and migrate to lymph nodes, as well as tumor cells.
“The idea behind this nanoparticle is to try and direct the delivery of the gadolinium contrast agent to lymph nodes that have cancer so that they show up brighter on the MRI than healthy lymph nodes,” Trac added.
Together with its significant clinical use in detecting metastases for the first time after an initial cancer diagnosis, the diagnostic tool will also help doctors monitor cancer recurrence.
Chung noted, “Just say a primary tumor has been removed, but perhaps they didn't get all of it, or the cancer comes back and it's metastatic for the second time. Recurrent metastasis is much harder to detect and can lead to worse outcomes for the patient.”
Hitching a Ride to Light Up Cancer
The nanoparticles target a protein called C-C chemokine receptor 2 (CCR2), produced by cancer cells. The particles “hitchhike” onto the body’s immune cell monocytes, which express the same receptor in response to malignancy. The monocytes then transport the particles to the lymph nodes, where they effectively highlight metastatic cancer cells and allow for more accurate diagnosis using MRI.
Chung stated, “The reason why this mechanism works, in addition to the targeting elements, is because our particle size is also very unique, and it can reach the lymph nodes. We found there's a size cut-off and our particle type is able to pass into the lymph nodes and target cancer cells that have gotten there, along with the monocytes that express this receptor.”
The method has game-changing implications for the early identification of cancer metastases in lymph nodes. Previously, metastasis could only be detected by increasing lymph node size; however, the new Chung Lab particles may lead to MRI contrast agents which could detect metastatic cells in lymph nodes that seem normal. In mouse model examinations, researchers revealed that the particles boosted the MRI signal observed by up to 50%.
Chung concluded, “The particles are amplifying the signal, and we can see that at points where the lymph nodes haven’t yet changed in size, and the metastasis is very early. We are providing this benefit where, clinically, you wouldn't be able to see metastasis at all.”
The study team’s next step is to bring their findings closer to clinical uses for MRI contrast agents. The study has been submitted to the National Institutes of Health’s Nanoparticle Characterization Laboratory, where it will be assessed and validated by a third party before proceeding to human trials.
Journal Reference:
Trac, N., et. al. (2024) MRI Detection of Lymph Node Metastasis through Molecular Targeting of C–C Chemokine Receptor Type 2 and Monocyte Hitchhiking. ACS Nano. doi:10.1021/acsnano.3c09201.
Source: https://www.usc.edu/