Bruker’s NanoWizard® 4 XP BioScience atomic force microscope integrates atomic resolution, a wider scan range of 100 µm, and rapid scanning with rates of up to 150 lines/second into a single system.
The microscope has been engineered to offer the highest thermal and mechanical stability on inverted optical microscopes while performing long-duration experiments on samples. The samples range from single molecules to living tissues and cells.
The NanoWizard® 4 XP BioScience comes with:
- Fast scanning option with rates of up to 150 lines/second
- DirectOverlay™ 2 software for data correlation and flawless integration with sophisticated fluorescence microscopy platforms
- PeakForce Tapping® enables easy imaging
- V7 Software with new, innovative workflow-based user interface
- NestedScanner Technology allows high-speed imaging of surface structures measuring up to 16.5 µm with excellent stability and resolution
- Vortis™ 2 controller for lowest noise levels and high-speed signal processing
- New tiling functionality for automated mapping of large sample areas
Automated Mapping of Large Sample Areas with New Tiling Functionality
The HybridStage™ or Motorized Precision Stage revolutionizes experiments by allowing direct access to a huge sample area, through motorized, automated movement to chosen positions, mapping regions, and grids. The experiment can be started with the DirectOverlay 2 optical calibration, and then a region can be selected for optical tiling up to millimeters in size.
Accurate motor movements bring the entire sample into view all by itself, enabling regions and features to be easily selected for further analysis. A single click enables navigation from point to point, or MultiScan experiments automate a string of measurements at chosen points.
Perfect Optical Integration Provides True Correlative Microscopy
Thanks to its fast imaging capabilities and exclusive tip-scanning technology, the NanoWizard® 4 XP is best suited for leveraging the synergy between super-resolution microscopy and AFM. The instrument is compatible with a broad array of platforms, for example, those from Leica (STED), Nikon (SIM, STORM), Zeiss (PALM/STORM, SIM), Abberior (STED), and PicoQuant (STED).
The AFM head’s 980-nm laser option enables concurrent use of focus stabilization systems and optical microscopes, which is crucial for long-duration experiments and prevents conflicts with spectroscopy or fluorescence measurements.
Outstanding Quantitative Data from Molecules, Cells, and Tissues
QI™ Advanced is based on real force curves, enabling both amazing speeds and resolution for applications that range from single molecules to living cells. The quantitative data enables fast and accurate analysis of biochemical or mechanical interactions. For example, the localization of binding sites, directly overlaid with fluorescent labeling and topography using Molecular Recognition Imaging.
Multiple models for modulus fitting are included in the state-of-the-art batch processing options, thereby divulging surface topography at zero force using Contact Point Imaging (CPI).

 Read Next: Extreme Performance AFM Microscopy
Read Next: Extreme Performance AFM Microscopy
New Workflow-Based User Interface Redefines User-Friendliness
The interface of the innovative V7 software guides users through the workflow to intuitively set up experiments. It enables even users with very less AFM experience to advance confidently to produce high-quality data. Every stage of the setup and functioning acts as an improved desktop that brings together all the important information with a single click.
- Status feedback for alignment and setup
- Context-sensitive on-screen help
- Single-click probe calibration
- Efficient, task-based experiment selection
- Instant overview of crucial data
- Rapid access to recently used and favorite experiments
- User management for multi-user settings such as imaging facilities
Key Features
- New workflow-based user interface for ergonomics and ease of operation
- Fast scanning option with rates of up to 150 lines/second for monitoring dynamic processes
- Optimized DirectOverlay 2 mode for most accurate correlative microscopy
- Includes Bruker’s unique PeakForce Tapping® as standard
- New Vortis 2 controller with most advanced position sensor readout technology and high-speed, low-noise DACs
- Atomic lattice resolution on inverted microscopes with a wider scan field of 100 × 100 × 15 µm3
- Highest upgradeability and flexibility with a wider range of accessories and modes
- Innovative tiling functionality enables automated mapping of wide sample areas in combination with the HybridStage™
NanoWizard 4 XP BioScience: AFM with Super-Resolution Compatibility

The image shows atomic lattice of mica in buffer, taken in closed-loop on an inverted microscope.

The image shows tailored DNA origami frames imaged in TAE 10 mM MgCl2 buffer on mica. Sample courtesy of R. Willaert, VUB, Brussels (BE). Scan field: 125 nm; Height range: 4.4 nm; Scan speed: 150 lines/second.
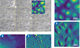
The images show living Vero cells in cell culture medium at 37 °C in PetriDishHeater™. (1) Optical tiling with 5 × 6 phase contrast images covering a 630 µm × 450 µm region. (2–5) Zoom into region scanned with AFM showing 100 μm × 100 μm scan (height range 5 μm) and inset 15 μm × 15 μm (height range 2 μm) scan topography images using PeakForce Tapping®. The feedback correction signal images highlight the surface membrane features, particularly in the zoomed image. Microvilli dominate the center of the cell, with membrane ruffles at the cell boundary.

The images show a STED and AFM experiment of living A549 cells imaged at 37 °C in medium. (1) STED image of microtubules labeled with silicon rhodamine overlaid with AFM topography. (2) AFM QI topography image at 240 pN imaging force (height range 3.5 μm). (3) Corresponding QI Young’s modulus image (z range 100 kPa).

NanoWizard® 4 XP setups on Zeiss LSM 880 confocal microscope with Airyscan (1), with Upright Fluorescence Microscope (UFM) Kit for tissues or other large samples such as organs (2), with BioMAT Workstation for high NA optics and Zeiss Axio Imager (3), with TopviewOptics (4) and on Olympus with PicoQuant MicroTime 200 STED (5).

Single-cell force spectroscopy measurements using the CellHesion module with an increased z range of 100 µm, showing the detachment force curves of a single A549 cell from fibronectin (FN) and from bovine serum albumin (BSA) coated culture dishes. Note that the detachment of the cell from fibronectin results in a very large pulling range of 77 µm.

(1–2) Stiffness mapping of non-cancerous human cervix tissue with a HybridStage™. The inset in the fluorescently labeled (Hoechst) slab was used for mapping of a 5 × 4 quadrant area of 1000 μm × 800 μm with overlaid composite Young’s modulus map shown in the middle panel. The representative topography channel from an individual 200 μm × 200 μm channel is given in the right panel. Sample courtesy of Dr T. Fuhs and Prof. J.A. Käs, University of Leipzig, Germany.