Photothermal therapies utilize photosensitizing agents to convert applied light into heat. This release of energy results in localized damage to targeted cells or other therapeutic effects.
Plasmonic nanoparticles are new candidates for photosensitizing agents which have gained attention as the optical properties (Ops) of the particles can be widely tuned. The size and shape of particles can be controlled, meaning formulations can be tailored to efficiently absorb light at specific wavelengths and convert it into heat.
Directing a laser into a solution of gold nanorods produces a rapid increase in the solution temperature, as photons are absorbed by the nanorods and converted to heat which is transferred to the surrounding fluid.
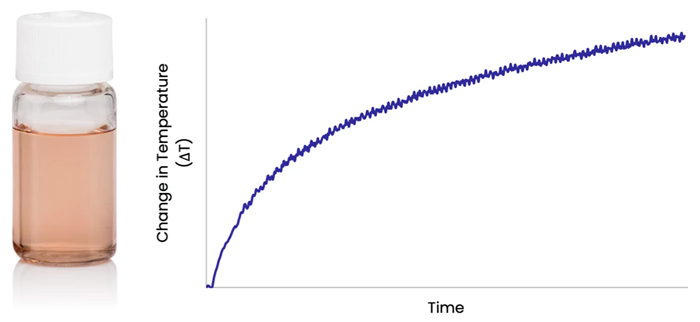
Figure 1. Image Credit: nanoComposix, Inc.
Near-infrared (NIR) light is often used for in vivo applications in photothermal therapies. In portions of NIR, biological tissues are relatively transparent, which means light can penetrate deeply without being absorbed and causing damage.
The OPs of nanoparticles can be adjusted to cross over with these biotransparent regions to absorb applied NIR light. Figure 2 demonstrates how the optical response of different types of nanoparticles can be altered to absorb NIR light within the biotransparent window.
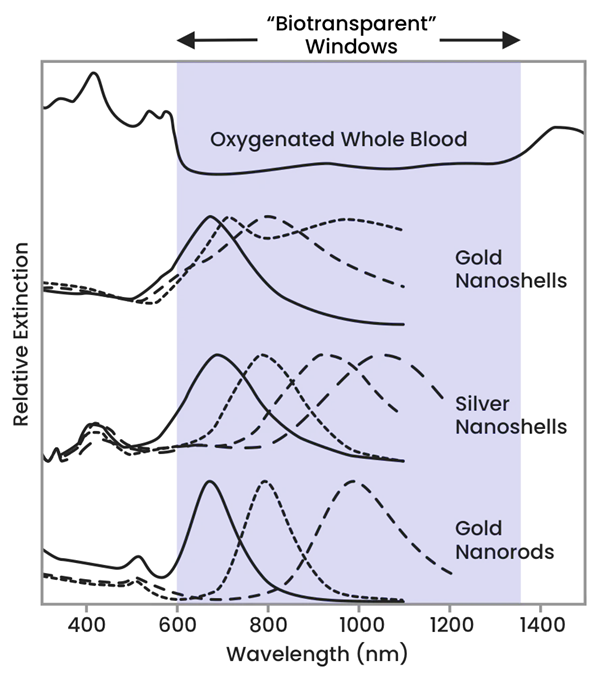
Figure 2. Image Credit: nanoComposix, Inc.
The photothermal effect generates heat which is highly localized to the area surrounding the nanoparticle, causing targeted damage to surrounding cells. The nanoparticle surfaces can be passivated with biologically inert poly(ethylene glycol) (PEG), or functionalized with antibodies or other targeting molecules to selectively bind to cells.
nanoComposix offers a complete library of plasmonic nanoparticles that can be manufactured under ISO 13485:2016 and GMP/QSR conditions for the production of biocompatible materials suitable for clinical trials or commercial use.
Table 1. Source: nanoComposix, Inc.
| Particle |
Size (nm) |
Plasmon Peak
Wavelength Range (nm) |
Application
Areas |
| Gold Spherical† |
5–100 |
500–600 |
Topical/Injectable Photothermal Therapy |
| Gold Nanorods† |
5–25 |
600–1100 |
Topical/Injectable Photothermal Therapy, Bioassays, Imaging, Drug and Gene Delivery |
| Gold Nanoshells† |
145–195 |
660–980 |
Topical/Injectable Photothermal Therapy, Sensing, Drug and Gene Delivery |
| Silver Nanoplates‡ |
40–150 |
500–1300 |
Topical Photothermal Therapy, Molecular Detection |
* Catalog nanoparticles from nanoComposix are intended for Research Use Only.
† hydrodynamic diameter measured by Dynamic Light Scattering (DLS)
‡ total diameter measured by Transmission Electron Microscopy (TEM)
Selection of Nanoparticles
Choosing nanoparticle type and surface chemistry for photothermal applications depends on a range of factors, such as whether the particles will be used for nanoheating applications, topical or injectable therapies, or other in vitro uses.
Additional considerations include the formulation used for storage and delivery, the environment to which the particles will be exposed, and the desired OPs (in combination with the illumination source).
Nanoparticle Material and Morphology
For photothermal applications, gold and silver nanoparticles are usually chosen due to their ability to alter OPs through visible and NIR regions of the spectrum to overlay with common laser wavelengths by varying particle size and shape.
Figure 3 displays the range across which the plasmon resonance of different nanomaterials can be tuned.
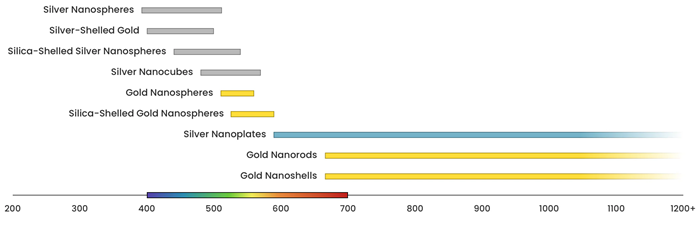
Figure 3. Image Credit: nanoComposix, Inc.
The atmosphere that the particles will be exposed to is important to consider when maintaining the stability of the particles and OPs over a given time period. Compared with silver-based nanoparticles, gold-based nanoparticles are commonly more inert over a wider range of solution conditions.
Silver particles may etch quickly when exposed to low-pH environments or salt, leading to undesirable changes to the particle OPs or the release of free silver ions if coatings around the particles are not used to enhance stability.
For these reasons, silver nanomaterials are commonly selected for topical applications, while gold nanomaterials may be used for injectable and topical treatments.
Scattering and Absorption
In addition to the general alteration of the plasmon resonance wavelength based on morphology and composition, particles' wavelength-dependent scattering and absorption responses can also be adjusted.
The figure on the left (Figure 4) displays the total extinction (scattering plus absorption) measured for a solution of gold nanoshells. The figure on the right indicates the calculated extinction for a solution of gold nanoshells, together with the calculated scattering and absorption components making up the total extinction.
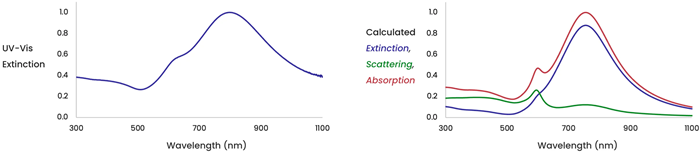
Figure 4. Image Credit: nanoComposix, Inc.
In photothermal applications, the OPs' absorption component converts light into heat energy. The scattering contribution can distribute light energy throughout a region and increase the pathlength of light through a sample, providing more opportunities for absorption.
Nanoparticle OPs can be fine-tuned to boost efficiency in particular applications.
Nanoparticle Surface Modification
Implementing nanoparticles for photothermal applications necessitates the selection of appropriate particle size, shape, and metal type, as well as chemistry and targeting ligands.
nanoComposix can modify the surface of many nanoparticles with specific functional groups, inorganic coatings, biocompatible polymers, and biomolecules, such as proteins, antibodies, and oligonucleotides. Table 2 summarizes some of the common surface modifications utilized by nanoComposix for photothermal applications.
Table 2. Source: nanoComposix, Inc.
| . |
. |
 |
Polyethylene glycol (PEG) ligands offer a high degree of biocompatibility, can improve stability in high salt environments, and reduce non-specific interactions. PEG ligands can be covalently bound to the metal surface to produce stable coatings. |
 |
Silica shells act as partial barrier between the core particle and surrounding environment, providing compatibility with a wide range of solvents and improving thermal stability. The silica surface is easily modified to introduce functional groups or polymers on the surface or to attach targeting biomolecules. |
 |
Antibodies, peptides, oligos and other targeting biomolecules can be attached to many metal nanoparticle surfaces by physisorption (passive conjugation) or covalent attachments (EDC/NHS chemistry, click reagents, or other coupling methods). |
Photothermal Treatments and Applications
Hyperthermia
Nanoparticle-based hyperthermia is a method in which nanoparticles are locally or systemically administered and then stimulated by an external energy source to generate heat, which selectively damages local tumor cells or tissues.
Together with surgery, chemotherapy, or radiotherapy, hyperthermia is an efficient adjuvant cancer therapy.
The application of metal nanoparticles can offer some advantages compared to traditional approaches.
The passivation of metal nanoparticles with biocompatible surfaces, including polyethylene glycol (PEG), can achieve long circulation times. This permits the accumulation of particles in tumors through the enhanced permeability and retention (EPR) effect or by functionalizing the particles with molecules that target cancer.
As gold is inert and does not react with biological tissues, there is less concern regarding the toxicity of gold nanoparticles compared with some other therapeutic agents. However, research and clinical trials are ongoing to understand potential dangers further.
The absorption cross-section of nanoparticles can be several orders of magnitude larger than the organic dye molecules, which may be used for absorption, further advancing the particles’ efficacy in absorbing light and converting it to heat.
Drug and Gene Delivery
Nanoparticles can be functionalized and loaded with active pharmaceutical ingredients (APIs) to act as promising nanocarriers for the targeted delivery of drugs or genes. Their load can be released in the designated location through plasmonic heat generation after NIR radiation irradiation, enabling another photothermal therapy format.
Gold nanoparticles can be covered or loaded with various compounds, including hyaluronic acid, polymers (polyethylene glycol, poly(N-isopropylacrylamide), proteins (ovalbumin, biotin), doxorubicin, folate, DNA and RNA (siRNA and shRNA), and can thus offer a flexible platform for delivering therapeutic cargo.
Applications in Dermatology and Topical Therapeutics
Gold and silver nanoparticles are utilized in photothermal therapies for treating skin conditions, such as acne and rosacea, along with other dermatology applications related to hair removal or liposuction and skin cancer.
Gold-based particles are typically only used for in vivo applications, whereas gold- and silver-based nanoparticles can be used for topical applications.
Topical formulations using gold or silver nanoparticles can be utilized similarly to treat acne and increase the efficiency of laser hair removal. In such applications, a gel containing nanoparticles with adjusted optical absorption is applied to the skin, and ultrasonic energy is used to drive the particles into the pores.
The heating of the nanoparticles can cause targeted heating and damage to the sebaceous gland, reducing the production of sebum that causes acne. Heat can also damage the follicle to remove unwanted hair.
The surface functionalization, composition, and size of the nanoparticles are crucial for attaining efficient penetration into the pores and determining the efficiency of the treatment.
Local heating caused by gold nanoparticles injected into fat tissue can also improve the efficiency of liposuction procedures and potentially improve patient recovery time.
Biological Cryopreservation
Cryopreservation allows viable cells and tissues to be preserved over time in a frozen, vitrified state. Cell ice formation is usually lethal during the transition between biological and cryogenic temperatures. Therefore, cooling and heating rates must be quick enough to avoid the formation of ice crystals.
Although injecting cryoprotectant agents can reduce ice formation, other approaches may be necessary to increase the cryopreservation success rate.
Preserving large aquatic embryos, such as in the genetic banking of embryos for research or aquaculture, is an example of where such approaches are needed.
A collaboration between nanoComposix and Professor John Bischof (University of Minnesota) demonstrated that gold nanorods can be used as a platform for the cryopreservation of zebrafish embryos, where the application of external convective heating during the rewarming process is limited by the large size of the embryos.
Injecting biocompatible gold nanorods into the tissue before freezing allowed an external laser to quickly heat the frozen embryos from within, considerably increasing the survival rate and producing embryos that developed into normal adults.
Such a technique has wide-ranging implications as a platform technology to preserve the germplasm of many systems, both vertebrate and nonvertebrate. It may be adapted to larger cell and small tissue systems.
The ability to preserve these germplasms will provide a vital tool for maintaining biodiversity while also upholding important genetic research models.
Plasmonic Heating and Thermocycling
In addition to being able to induce localized damage to local tissues for hyperthermia applications selectively, plasmonic nanoparticles can be used for in situ bulk heating of particle dispersions.
This method can overcome the relatively slow thermocycling time that limits the throughput of conventional polymerase chain reaction (PCR) techniques, which use bulky block heaters.
PCR in combination with LED or the laser irradiation of nanoparticle solutions has significantly reduced the time associated with each heating step, improving the DNA amplification rate.
Adding nanoparticles into amplification steps and other assays may improve transportability and increase the throughput of point-of-care applications.
References and Further Reading
Cui, X., et al. (2023). Photothermal Nanomaterials: A Powerful Light-to-Heat Converter. Chemical Reviews, 123(11), pp.6891-6952.
Hirsch, L. R., et al. (2003). Nanoshell-mediated near-infrared thermal therapy of tumors under magnetic resonance guidance. Proceedings of the National Academy of Sciences, 100(23), pp.13549-13554.
Riley, R. S. and Day E. S. (2017). Gold nanoparticle‐mediated photothermal therapy: applications and opportunities for multimodal cancer treatment. Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology, 9(4), e1449.
Mendes, R., et al. (2017). Photothermal enhancement of chemotherapy in breast cancer by visible irradiation of gold nanoparticles. Scientific Reports, 7(1), pp.10872.
Singh, P., et al. (2018). Gold nanoparticles in diagnostics and therapeutics for human cancer. International Journal of Molecular Sciences, 19(7), pp.1979.
Chatterjee, D. K., Diagaradjane, P. and Krishnan, S. (2011). Nanoparticle-mediated hyperthermia in cancer therapy. Therapeutic Delivery, 2(8), pp.1001-1014.
Liu, J., et al. (2015). Gold nanorods coated with mesoporous silica shell as drug delivery system for remote near-infrared light‐activated release and potential phototherapy. Small, 11(19), pp.2323-2332.
Kawano, T., et al. (2009). PNIPAM gel-coated gold nanorods for targeted delivery responding to a near-infrared laser. Bioconjugate Chemistry, 20(2), pp.209-212.
Paithankar, D., et al. (2015). Ultrasonic delivery of silica–gold nanoshells for photothermolysis of sebaceous glands in humans: Nanotechnology from the bench to clinic. Journal of Controlled Release, 206, pp.30-36.
Friedman, N., et al. (2021). Physical properties of gold nanoparticles affect skin penetration via hair follicles. Nanomedicine: Nanotechnology, Biology and Medicine, 36, pp.102414.
Sheng, W., et al. (2018). A Single-Blind Study Evaluating the Efficacy of Gold Nanoparticle Photothermal-Assisted Liposuction in an Ex Vivo Human Tissue Model. Aesthetic Surgery Journal, 38(11), pp.1213-1224.
Khosla, K., et al. (2017). Gold nanorod induced warming of embryos from the cryogenic state enhances viability. ACS Nano, 11(8), pp.7869-7878.
Daly, J., et al. (2018). Successful cryopreservation of coral larvae using vitrification and laser warming. Scientific Reports, 8(1), pp.15714.
Blumenfeld, N. R., et al. (2022). Multiplexed reverse-transcriptase quantitative polymerase chain reaction using plasmonic nanoparticles for point-of-care COVID-19 diagnosis. Nature Nanotechnology, 17(9), pp.984-992.
Cheong, J., et al. (2020). Fast detection of SARS-CoV-2 RNA via the integration of plasmonic thermocycling and fluorescence detection in a portable device. Nature Biomedical Engineering, 4(12), pp.1159-1167.

This information has been sourced, reviewed and adapted from materials provided by nanoComposix, Inc.
For more information on this source, please visit nanoComposix, Inc.