Feb 10 2011
Coating a lattice of tiny wires called Nanonets with iron oxide – known more commonly as rust – creates an economical and efficient platform for the process of water splitting, an emerging clean fuel science that harvests hydrogen from water, Boston College researchers report in the online edition of the Journal of the American Chemical Society.
Assistant Professor of Chemistry Dunwei Wang and his clean energy lab pioneered the development of Nanonets in 2008 and have since shown them to be a viable new platform for a number of energy applications by virtue of the increased surface area and improved conductivity of the nano-scale netting made from titanium disilicide, a readily available semiconductor.
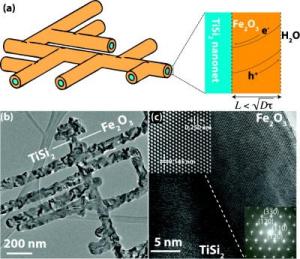 Boston College researchers have tested their Nanonet design as a platform for clean energy applications. Most recently, coating the highly conductive titanium disilicide core (a) with hematite, the mineral form of iron oxide, or rust, dramatically improved the performance of the material at its fundamental state. Transmission electron microscopy image (b) shows the structural complexity of the Nanonet and additional images ( c ) detail the hematite Nanonet spacing, as well as the electron diffraction pattern of hematite (lower right corner).
Boston College researchers have tested their Nanonet design as a platform for clean energy applications. Most recently, coating the highly conductive titanium disilicide core (a) with hematite, the mineral form of iron oxide, or rust, dramatically improved the performance of the material at its fundamental state. Transmission electron microscopy image (b) shows the structural complexity of the Nanonet and additional images ( c ) detail the hematite Nanonet spacing, as well as the electron diffraction pattern of hematite (lower right corner).
Wang and his team report that coating the Nanonets with hematite, the plentiful mineral form of iron oxide, showed the mineral could absorb light efficiently and without the added expense of enhancing the material with an oxygen evolving catalyst.
The results flow directly from the introduction of the Nanonet platform, Wang said. While constructed of wires 1/400th the size of a human hair, Nanonets are highly conductive and offer significant surface area. They serve dual roles as a structural support and an efficient charge collector, allowing for maximum photon-to-charge conversion, Wang said.
"Recent research has shown that the use of a catalyst can boost the performance of hematite," said Wang. "What we have shown is the potential performance of hematite at its fundamental level, without a catalyst. By using this unique Nanonet structure, we have shed new light on the fundamental performance capabilities of hematite in water splitting."
On its own, hematite faces natural limits in its ability to transport a charge. A photon can be absorbed, but has no place to go. By giving it structure and added conductivity, the charge transport abilities of hematite increase, said Wang. Water splitting, a chemical reaction that separates water into oxygen and hydrogen gas, can be initiated by passing an electric current through water. But that process is expensive, so gains in efficiency and conductivity are required to make large-scale water splitting an economically viable source for clean energy, Wang said.
"The result highlights the importance of charge transport in semiconductor-based water splitting, particularly for materials whose performance is limited by poor charge diffusion," the researchers report in the journal. "Our design introduces material components to provide a dedicated charge transport pathway, alleviates the reliance on the materials' intrinsic properties, and therefore has the potential to greatly broaden where and how various existing materials can be used in energy-related applications."
Source: http://www.bc.edu/