This novel idea is based on the fact that cancer cells can meet their demand for iron by enhancing iron metabolism. Although iron can enable the biological activity of cancer cells, the abundance of this element can potentially result in ferroptosis – cancer cell death.
What is Ferroptosis?
Researchers refer to the proposed idea of ferroptosis through mediating iron-dependent peroxidation, which was first explored in 2021. This process leads to cell membrane collapse due to the overwhelming peroxidation level – the oxidative degradation of lipids.
The abundance of lipid peroxidation alters the morphological structure of the cell. Compared to other programmed cell death processes, ferroptosis can be seen by a distinct characterization, along with changes in mitochondrial morphology. Comprehending this process can aid researchers in developing drugs that enhance the treatment of cancer.
The process of ferroptosis consists of three critical steps: depletion of antioxidant defenses, accumulation of iron, and lipid peroxidation.
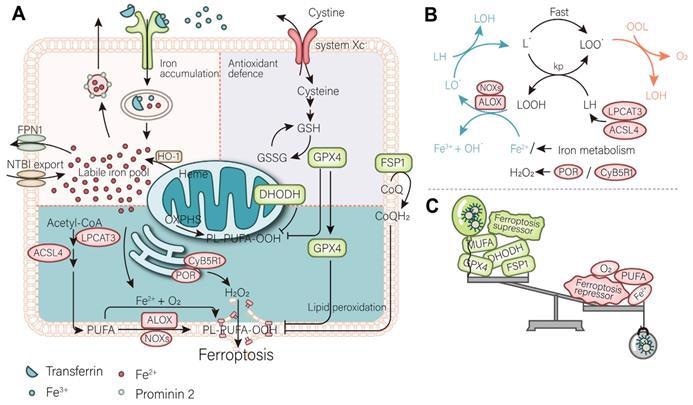
Mechanism of ferroptosis. (A) A mutually independent antioxidant defense axis composed of xCT-GPX4, FSP1, DHODH, protects cells against ferroptosis by antagonizing lipid peroxidation. At the same time, the entry and exit of intracellular iron as well as changes in availability regulate the sensitivity of cancer cells to ferroptosis. Lipid metabolism and PUFA synthesis provide substrates for the occurrence of ferroptosis. A series of oxidases become promoters of lipid peroxidation and the Fenton reaction. (B) An illustration of the role of these proteins in the detailed mechanism of lipid peroxidation is partially shown. (C) In general, the imbalance of intracellular ferroptosis-inducing and -inhibiting factors leads to ferroptosis, while the purpose of nanomaterials is to increase the weight of ferroptosis-inducing factors and reduce that of ferroptosis-inhibiting factors (such as antioxidant defenses). Image Credit: Luo, L., et al
Depletion of Antioxidant Defences
There are three antioxidant defense systems located in different areas of the cells. Each has independent functions, but all have complementary roles in inhibiting the process of ferroptosis. These separate systems of defense, known as GPX4, FSP1, and DHODH, require further understanding for use as targets for drug development towards cancer treatment.
Through disabling the antioxidant systems in place to defend and protect the lipid membrane against reactive oxygen species (ROS), an overwhelming and abundant level of lipid peroxidation can cause the cell membrane to collapse and result in cell death.
Accumulation of Iron
Additionally, for ferroptosis to occur, iron accumulation is required, triggered first by divalent iron irons that stimulate sensitivity to ferroptosis.
Cancer cells have a higher requirement of iron than normal healthy cells, so transferrin receptor expression is higher in cancer cells. Transferrin is a protein that can bind extracellular ferric ions that can be delivered to cancer cells through transferrin receptors.
Transferrin receptor expression is also upregulated during ferroptosis. As this receptor is of critical use for cancer cells, it can be a critical target for cancer treatment.
In vitro studies observing iron levels have found that ferroptosis is stimulated when iron overload occurs. In vivo studies have also supported this evidence, confirming that a diet high in iron can increase the efficiency of cancer treatment focusing on ferroptosis.
Lipid Peroxidation
Lipid peroxidation is the result of iron accumulation, where ferrous ions react with the phospholipid cell membrane to start the critical lipid peroxidation chain reaction.
The oxidative degradation of lipids is critical for destroying the cell membrane and programmed cell death; this final step would be monumental for a targeted cancer treatment therapy focused on ferroptosis.
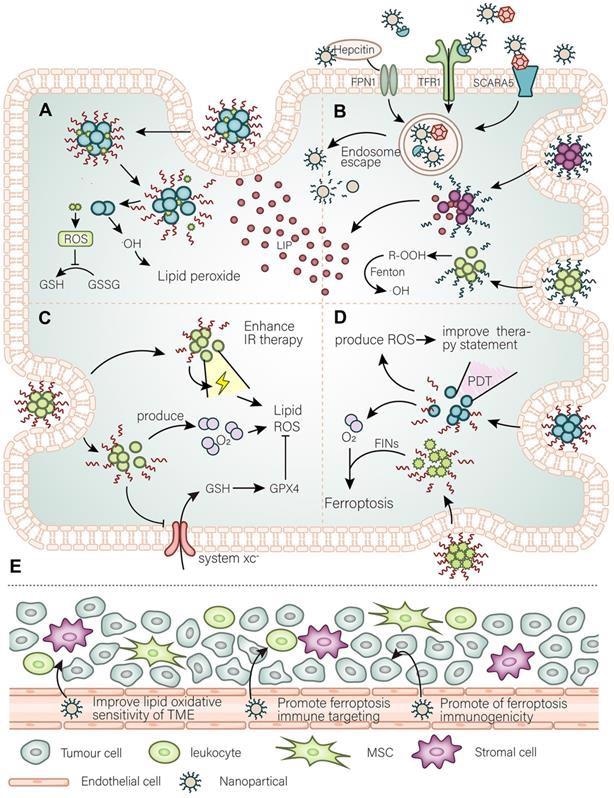
The main purpose of nanomaterials to target ferroptosis. (A) A general simple strategy for the development of nanomaterials is to improve the targeting of ferroptosis inhibitors to cancer tissues and modify the intracellular oxidative environment. (B) At the same time, the high demand for iron by cancer cells is the basis for the development of nanomaterials to target cancer cells, such as using Tfr to further expand or utilize the abundant unstable iron pool in the cancer cells. (C) By developing a strategy for releasing O2 as well as weakening of antioxidant defense systems to promote the damaging effect of ionizing radiation (IR) and further promote IR-induced ferroptosis. (D) Photodynamic triggered nanomaterials improve the treatment quality by adding an oxygen-releasing strategy combined with FINs. (E) In the TME, nanomaterials promote cancer cell immunogenic ferroptosis, promote infiltration of immune cells, and adjust the lipid balance of the immune microenvironment to curb the invasion and spread of cancer cells. Image Credit: Luo, L., et al
Nanomaterials for Ferroptosis
Nanoscale materials are beneficial for drug therapies, and the use of nanotechnology enables functionalization, which can further enhance the specificity and reduce the impact on healthy cells.
The impairment of antioxidant defenses within cancer cells would help to sensitize them for the process of ferroptosis to occur. Triggering this could involve the use of anticancer drugs which target solid tumors and can make them vulnerable to ferroptosis.
The loading of ferroptosis inducers within nanomaterials can be an effective therapy for cancer treatment. Utilizing nanocarriers can aid in disrupting the antioxidant defense system while also unloading anticancer drugs. This two-part targeting of cancer cells can assist in targeted therapies focused on ferroptosis.
Research focusing on such a process has already been undertaken, involving the development of a GSH-responsive sulfur dioxide polymer prodrug intended as a carrier for effective cancer treatment.
An approach like this would be useful, as sulfur dioxide can regulate the oxidative levels in tumors. With the use of GSH, or thiolyl, which releases sulfur dioxide, ROS is increased within the cell, and GSH is decreased.
This route to target cancer cells utilizes a drug external to the nanocarrier and can disrupt the sulfur dioxide balance in tumors, which ultimately disrupt the oxidative levels, disabling the antioxidant defenses, eventually leading to cell death.
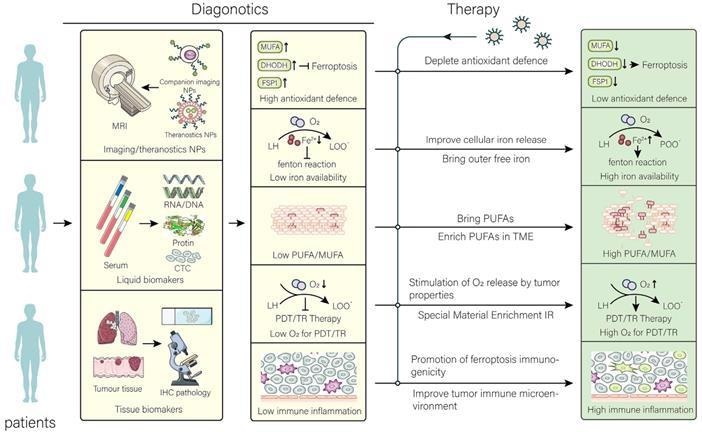
Application and perspective in nanomaterials for targeting ferroptosis. In the clinic, the aim of nanomaterials is to overcome the limitations of different tumors and their microenvironment. After determining the limitations that are not conducive to treatment by different diagnostic tools, the targeted treatment is performed using the corresponding nanomaterials to achieve the best efficacy at the lowest cost. The common resistance of tumors to ferroptosis has been overcome by therapeutic strategies. Image Credit: Luo, L., et al
Future Therapies
The review illustrates the importance of future therapies that focus on the iron dependency of cancer cells which can be exploited to induce ferroptosis.
With membrane proteins for iron entry being upregulated in cancer cells, they can be used as targets for enhanced cancer therapy. Nanocarriers that target transferrin receptor 1 (TfR1) have been researched to target this critical receptor.
The use of transferrin can be seen to be a significant component for targeting cancer cells. As transferrin can cross the blood-brain barrier, nanomaterials that use transferrin can be utilized to create enhanced therapies for brain tumors.
Further research involving in vivo experimentation on how ferroptosis can be used safely in patients would be required. However, research into inducing programmed cell death with nanomaterials can be the start of an advanced therapy towards cancer treatment.
Continue reading: Manifesting Multidisciplinary Nanomedicine Research with the MMS.
Reference
Luo, L., Wang, H., Tian, W., Li, X., Zhu, Z., Huang, R., & Luo, H. (2021) Targeting ferroptosis-based cancer therapy using nanomaterials: strategies and applications. Theranostics, 11(20), 9937–9952. Available at: https://www.thno.org/v11p9937.htm
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.