The basics of nanomaterial morphological manipulation were explored in a paper recently published in the journal Nano Energy. Here, the impacts of topological adjustment on several rechargeable lithium cell compositions were investigated.

Study: Advances in and Prospects of Nanomaterials’ Morphological Control for Lithium Rechargeable Batteries. Image Credit: petrmalinak/Shutterstock.com
Researchers emphasized the advantages and disadvantages of various topologies, the problems of batteries based on nanomaterials, and their industrial prospects.
Lithium Rechargeable Cells
Energy storing is a major objective in expanding power management setup. Rechargeable Li batteries have progressed well beyond older kinds of cells, leading the electronic sector and, more lately, the electric vehicle (EV) space. Over the last three decades, the energy content of Li-ion batteries has nearly doubled, owing mostly to the usage of large-capacity multilayer Ni-rich cathodes.
For energy-intensive purposes, a LiFePO4 (LFP) cathode with high energy concentration has also been created. In the coming years, sophisticated large-capacity anode elements, such as silicon-based composites, are expected to surpass graphite anodes.
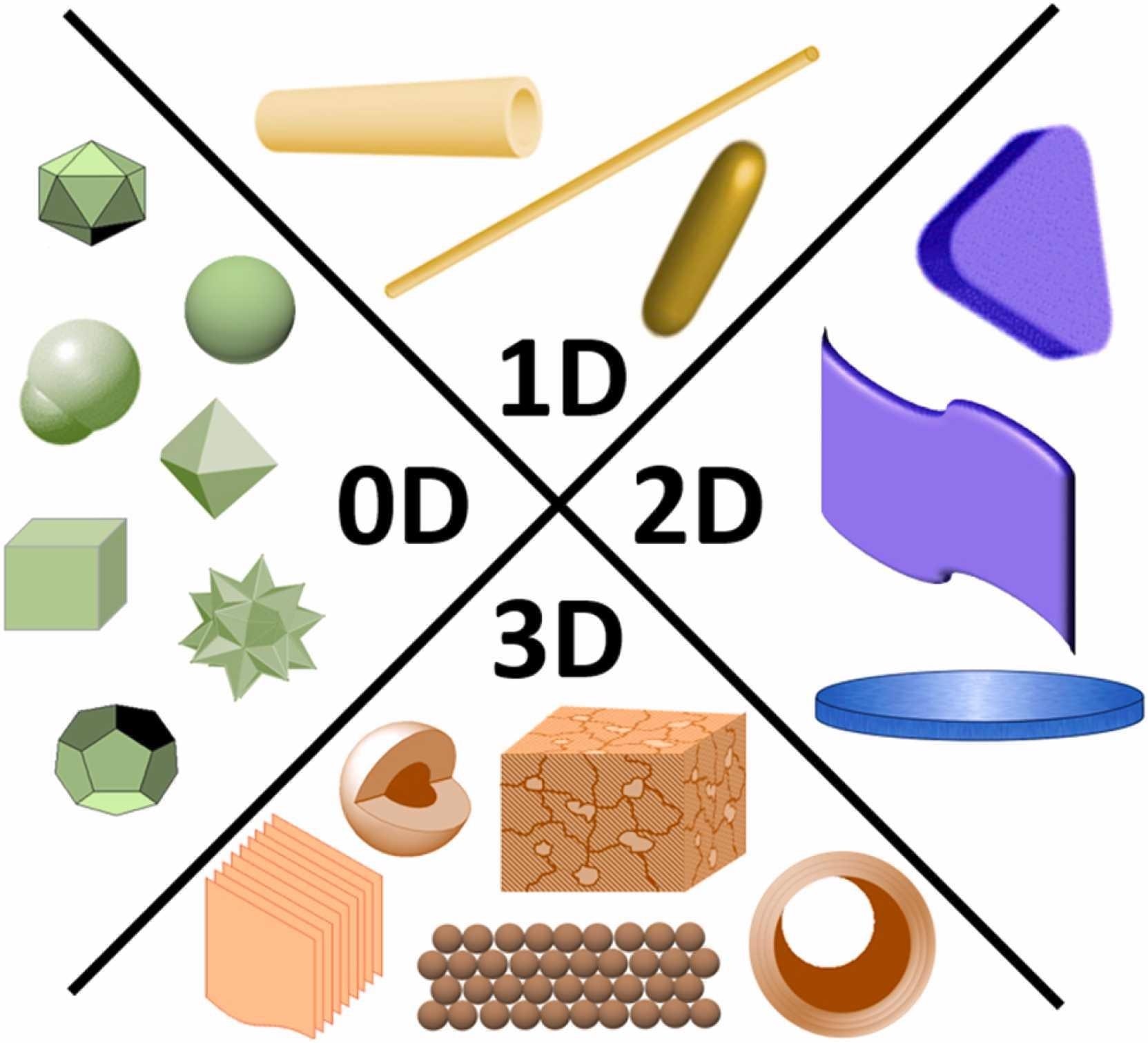
Schematic representation of different morphologies of nanomaterials. Based on their morphology, nanomaterials are classified into 0D, 1D, 2D, and 3D nanostructures. 0D nanomaterials have all dimensions in the nanoscale. 1D and 2D nanomaterials have one and two dimensions, respectively, beyond the nanoscale. 3D nanomaterials are microstructures with nanofeatures, such as hollow microstructures with nanoshells and nanoparticle-based micro assemblies. © AbdelHamid, A. A., Mendoza-Garcia, A., & Ying, J. Y. (2021).
Role of Nanotechnology in Li Battery Advancements
The creation of innovative and superior rechargeable lithium battery packs has been greatly aided by nanoscale structural processing. Nanomaterials' decreased dimensions may minimize the transportation pathways of lithium ions, allowing for quick reaction kinetics and high charging-discharging speeds.
Nanostructures are especially effective at tolerating architectural alterations during lithiation and de-lithiation processes due to their small size, which guards against material breakdown. The threshold diameters below which cracks are less prone to spread have been estimated, and the figures are in the nanometer range.
The greater specific area of nanomaterials contributes significantly to the increase in interlayer Faradaic reactions in cells and the lithium-ion flow across the electrolyte-electrode interface, resulting in increased capacity. Furthermore, the readily regulated degree of crystallinity in the majority of nanostructures offers stable passageways for charge movement and allows nanomaterials to tolerate cycle stresses, increasing the stability of the battery.
The importance of nanomaterials for rechargeable lithium batteries was covered in this study, as were the basic concepts employed in nanomaterial morphology control and the influence of various morphological structures on different rechargeable lithium battery makeups.
![Application of nanomaterials in Li rechargeable batteries. (a1) Graph showing the cumulative number of publications on Li rechargeable batteries using nanomaterials during 1995–2019 (Web of Science, accessed 20 May 2020). The light blue arrow shows the cumulative percentage of publications in the years 2000, 2010, and 2019, with representative images from publications in these years shown in (a2–a4), respectively. (a2) 100-200 nm CoO particles were reported as conversion-type anode for the first time [102]. (a3) A more developed composite of 10-30 nm Co3O4 particles on rGO sheets, used as a high-capacity anode [109]. (a4) A complex hybrid design comprising TiO2 nanotubes and SnS2 nanosheets with highly stable anode performance [110]. (b) Schematics (left) of 0D, 1D, 2D, 3D, and hybrid nanomaterials, with a comparison (right) between their advantages and disadvantages for Li rechargeable battery application.](https://www.azonano.com/images/news/ImageForNews_38432_16407733870328111.jpg)
Application of nanomaterials in Li rechargeable batteries. (a1) Graph showing the cumulative number of publications on Li rechargeable batteries using nanomaterials during 1995–2019 (Web of Science, accessed 20 May 2020). The light blue arrow shows the cumulative percentage of publications in the years 2000, 2010, and 2019, with representative images from publications in these years shown in (a2–a4), respectively. (a2) 100-200 nm CoO particles were reported as conversion-type anode for the first time [102]. (a3) A more developed composite of 10-30 nm Co3O4 particles on rGO sheets, used as a high-capacity anode [109]. (a4) A complex hybrid design comprising TiO2 nanotubes and SnS2 nanosheets with highly stable anode performance [110]. (b) Schematics (left) of 0D, 1D, 2D, 3D, and hybrid nanomaterials, with a comparison (right) between their advantages and disadvantages for Li rechargeable battery application. © AbdelHamid, A. A., Mendoza-Garcia, A., & Ying, J. Y. (2021).
Different Dimensionalities of Nanomaterials
Recently, researchers have created and synthesized a number of monodispersing nanostructured materials for a variety of uses. In 0D nanomaterials, otherwise referred to as nanoparticles, all dimensions are in the nanometer scale. 1D nanomaterials, such as nanotubes, nanorods, and nanowires, have one geometrical parameter outside of the nanometer scale.
2D nanoscale materials such as graphene, nanolayers, nanofilms, and nano-coats have two dimensions out of the nanometer level as well as plate-like architectures. Finally, 3D nanoscale materials are microscale structures with nanoscale features/architectures; examples include nanoparticle assemblies, hollow micro-/nanostructures, bundles of nanowires, and multiple nanoscale layers metallo-organic frameworks (MOFs).
How the Morphological Control of Nanomaterials Impacts Rechargeable Li Batteries
Nanomaterials have been intensively researched to improve the activity of lithium batteries. To increase material performance, 0D nanomaterials have been extensively used. However, more complicated architectures have been devised to increase performance or satisfy particular demands because of the fast advancements in energy storage. A broad range of 0D, 1D, 2D, 3D, and hybrid nanostructures are now used in various Li-battery chemistries.
Beyond direct Li storage, their function in Li batteries has evolved to accelerate electrochemical processes and store reaction intermediates. Morphology control has been a major characteristic utilized to provide nanomaterials with great adaptability to various lithium battery packs.
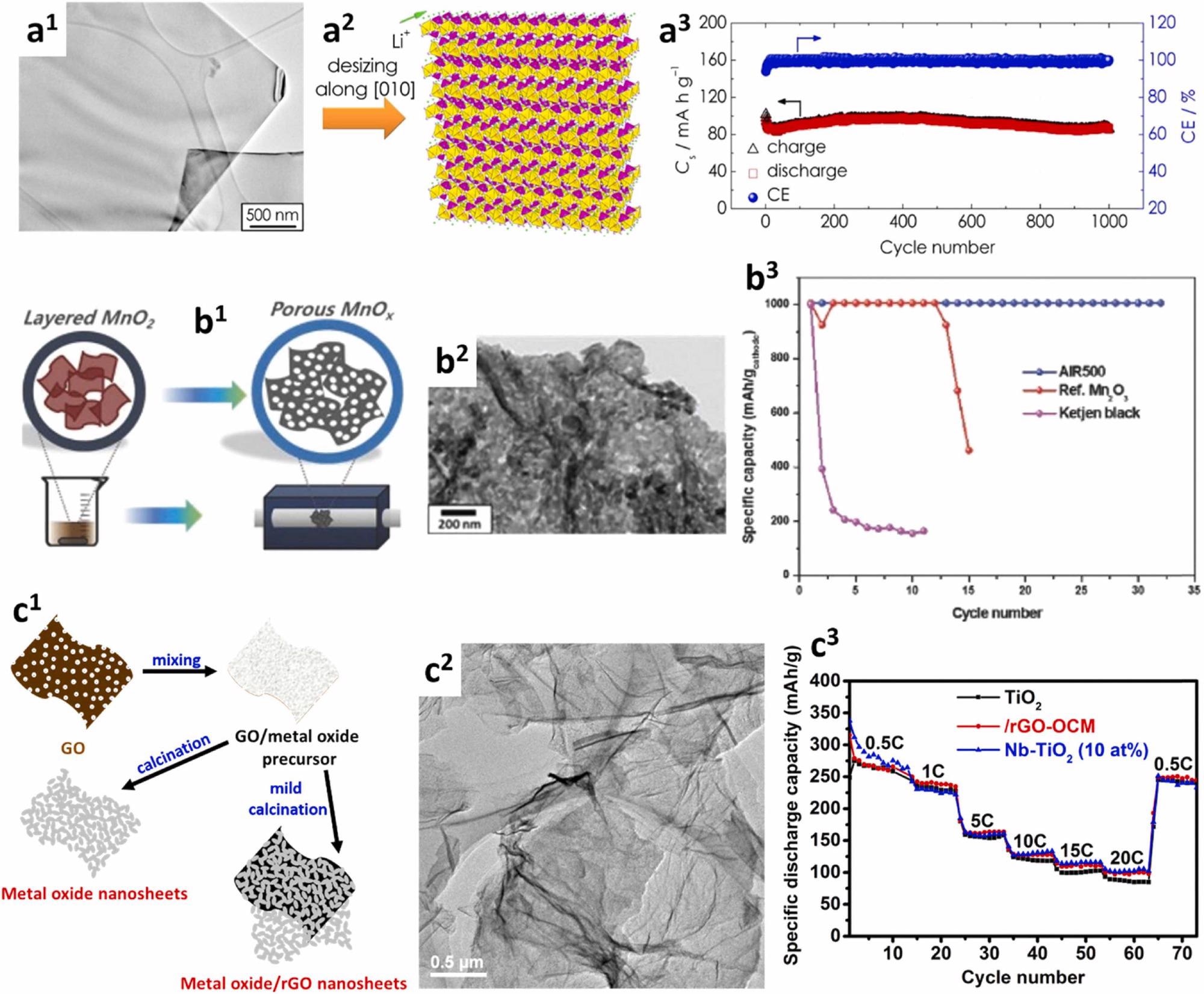
Application of 2D nanomaterials in Li rechargeable batteries. (a1–a3) LFP nanosheets confined along [010] direction for Li-ion battery cathode [67]. (a1) TEM image. (a2) Schematic showing short Li+ diffusion path along confined [010] direction. (a3) Cycling performance at 10 C. (b1–b3) Holey Mn2O3 nanosheets as Li-O2 battery catalyst [68]. (b1) Schematic depicting synthesis method. (b2) TEM image. (b3) Cycling performance at 0.2 A g–1 and a fixed capacity of 1000 mAh g–1. AIR500: holey Mn2O3 nanosheets. (c1–c3) Metal oxide nanosheets for Li-ion battery anode [50]. (c1) Schematic illustrating the synthesis methodology. (c2) Representative TEM image of TiO2 nanosheets. (c3) Rate capability of TiO2 nanosheets and their derivatives. © AbdelHamid, A. A., Mendoza-Garcia, A., & Ying, J. Y. (2021).
Challenges
It is critical to appreciate the inherent difficulties of their formulation and implementation. High electrolyte/electrode surface areas, irrespective of nanomaterial structure, may cause parasitic reactions during cycling, reducing the battery's lifespan.
The low tap density of some nanoparticles, on the other hand, may diminish the volumetric energy density. The widespread availability of nanomaterials for lithium batteries has not yet reached projections, owing to their difficult synthesis and expensive cost, particularly for 1D, 2D, and 3D nanostructures, and their inability to retain good performance at commercial standards.
Future Outlook
Nanomaterials have gained special consideration in lithium battery pack research because of their fast charge transfer pathways, volume change tolerance, improved electrolyte contact, strong catalytic properties, and varied performance. Structural control is crucial in producing nanostructures appropriate for their intended activities.
A wide variety of technologies are employed to create nanostructures with varying morphologies, including 0D, 1D, 2D, and 3D nanostructures. A broad range of morphologies may play different functions in lithium batteries, such as improved lithium storage, active material hosting, PS absorption and transformation, ORR, and OER.
Despite the problems that the large-scale use of nanoparticles for lithium batteries currently faces, a variety of nanostructures may be available in the market shortly.
Continue reading: A Solid State Electrode with Li-Ion Battery Applications.
Reference
AbdelHamid, A. A., Mendoza-Garcia, A., & Ying, J. Y. (2021). Advances in and Prospects of Nanomaterials' Morphological Control for Lithium Rechargeable Batteries. Nano Energy. Available at: https://www.sciencedirect.com/science/article/pii/S2211285521011095?via%3Dihub
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.