Researchers from RIKEN have discovered a quick and simple method for joining sulfur-containing functional groups with carbon nanobelts. Due to its intriguing properties, this new material shows promise for use in innovative optoelectronic devices. The study was published in the journal Nature Communications.
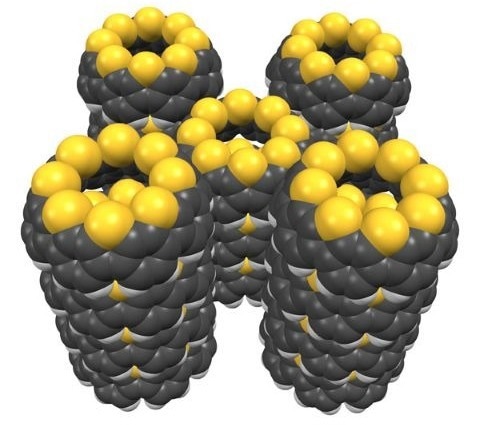 Five thiophene-fused nanobelts (yellow spheres: sulfur atoms; gray spheres: carbon atoms) in an organic solution. Image Credit: 2025 RIKEN Molecule Creation Laboratory
Five thiophene-fused nanobelts (yellow spheres: sulfur atoms; gray spheres: carbon atoms) in an organic solution. Image Credit: 2025 RIKEN Molecule Creation Laboratory
Tiny hollow cylinders made entirely of carbon atoms, known as carbon nanotubes, have been the subject of much interest since their discovery in 1991 and are being used in various fields, including electronics and medicine.
Cross-sectional slices of carbon nanotubes are called carbon nanobelts. They could serve as building blocks for carbon nanotubes and in electronic and optoelectronic devices. They were not synthesized until 2017 by a group headed by Kenichiro Itami, who is currently with the RIKEN Molecule Creation Laboratory, despite efforts dating back to the 1950s.
This accomplishment has spurred a rush to develop comparable nanobelts.
People realized that it is not a dream molecule, that it is possible to synthesize it. Therefore, many research groups in the world are now making a lot of different kinds of nanobelts after our demonstration.
Kenichiro Itami, Institute of Transformative Bio-Molecules, Molecule Creation Laboratory, RIKEN
Itami and his group have been creating new compounds based on carbon nanobelts. They have now included thiophene, a ring-shaped compound composed of four carbon atoms and one sulfur atom, with carbon nanobelts.
Thiophene's function-rich nature and intriguing semiconducting and fluorescence properties are the driving forces behind the combination of thiophene and carbon nanobelts.
Making the new molecule proved to be surprisingly simple. Itami said, “It is amazingly simple. It is just a single-shot reaction.”
Even Itami was surprised at how simple it was. “Hiroki Shudo, the first author of this paper, proposed the idea to me after reading a paper, saying, ‘I think we can do this. And then boom, it worked! That was a big surprise to me,” recalled Itami.
Another unexpected finding was that all of the nanobelts lined up with their sulfur sides facing up on a copper surface but down on a gold surface.
Itami noted, “That was really unexpected. We are now seeking to understand why it happens.”
However, the thiophene-fused nanobelts are more than just intriguing chemistry oddities; they may also find use in polar materials and optoelectronic devices.
Several research groups in other countries are extremely excited about using our molecule in devices and are looking to collaborate with us. So we are now sending the molecule to them to initiate international collaborations.
Kenichiro Itami, Institute of Transformative Bio-Molecules, Molecule Creation Laboratory, RIKEN
Other types of carbon nanobelts could be produced using the same synthesis method as the thiophene-fused nanobelts. Currently, Itami and his group are experimenting to see what other compounds they can create using it.
Journal Reference:
Shudo, H., et al. (2025) Thiophene-fused aromatic belts. Nature Communications. doi.org/10.1038/s41467-025-55896-w.