With epilepsy being a prevalent neurological disorder, it is important to comprehend its pathophysiology and undertake research that can aid the design of effective treatment. The introduction of nanotechnology has advanced medicine in various fields, and so its incorporation in the treatment of epilepsy would be perfectly suited.
Why Novel Epilepsy Treatments are Necessary
Epilepsy consists of a neurological disorder affecting the central nervous system through abnormal electrical activity within the brain areas such as the hippocampus and cortex. Subsequently, this results in symptoms such as frequent seizures, consisting of bursts of electrical activity in the brain that temporarily affects its normal function.
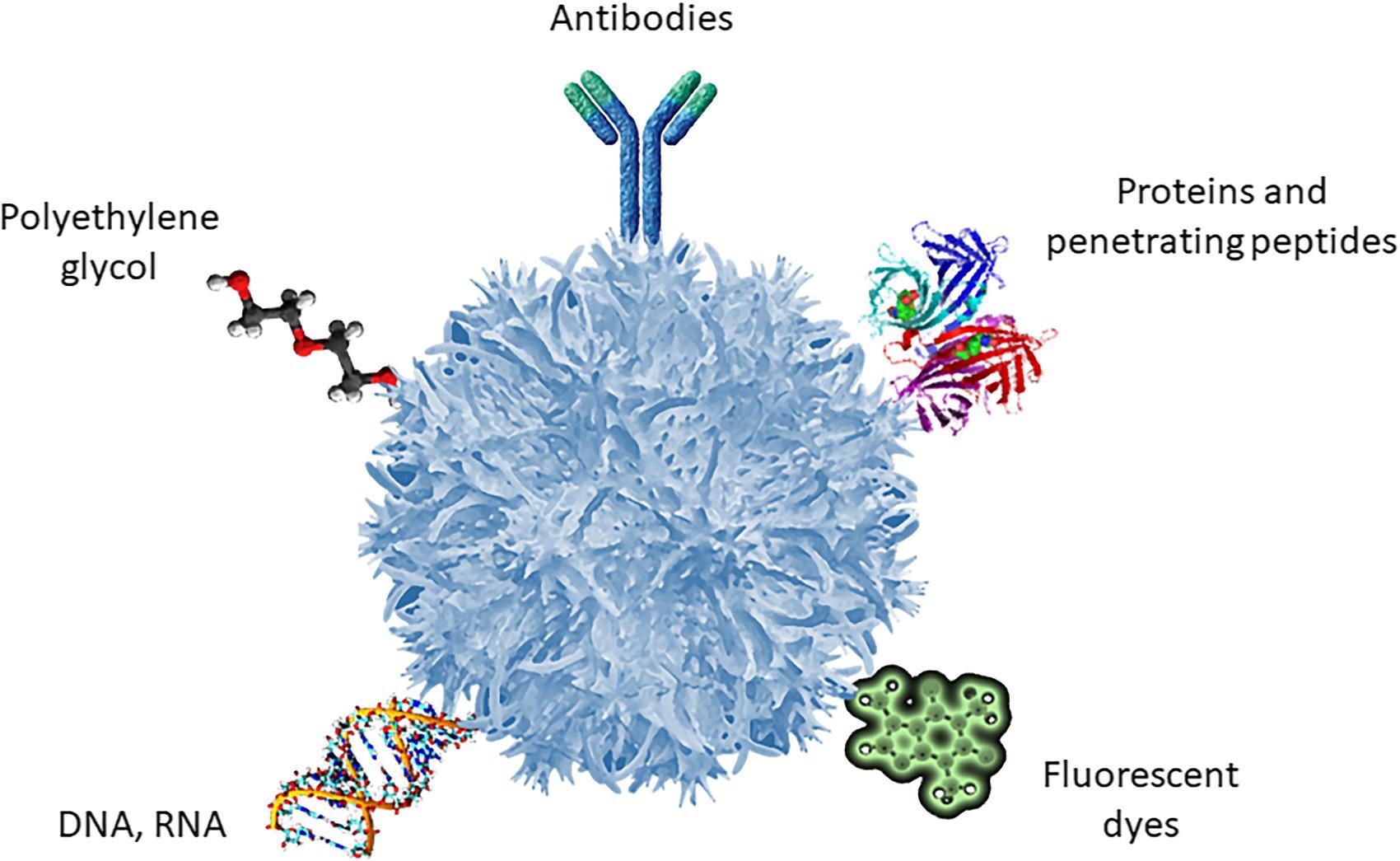
Common ligands of surface functionalization of polymeric nanoparticles. © Bonilla, L. et al (2021)
Neurodegeneration, which is a concern for this condition, can be biochemically described as having a large amount of calcium ions entering neurons – the main neurotoxic process that results in cell death and eventual degeneration of neurons.
The World Health Organization (WHO) has estimated that epilepsy affects more than 50 million people across the globe, with 5 million new diagnoses occurring annually.
Currently, while there are treatments that can help manage the disorder, there is no known cure, and antiseizure medication is not always effective.
This makes this field of medicine critical for researchers to develop innovative therapeutics. With the emergence of nanomedicine, the ability for advanced therapy can be a promising development.
Limitations of Current Antiseizure Treatments
Antiseizure medications (ASM) are an approach to manage the seizure symptoms of epilepsy, with the first ASM being used at the beginning of the 20th century and second-generation ASM drugs used as adjuncts in the 1990s. However, while there was an improvement seen in the effects, adverse effects were also seen.
Other limitations included having a narrow therapeutic margin and multiple interactions. This led to the development of third-generation ASM, which allowed more control of neuronal excitability to prevent a seizure.
These drugs work to decrease or modulate the excitability of neurons, which stops the expansion of electrical discharges in the brain. With various types of ASM available, the mechanism of action differs, such as some ASM targeting the excitatory synapse and working on blocking the activity of inotropic glutamate receptors including AMPA or NMDA receptors.
Other types of ASM available can target ion channels of the extrasynaptic membrane that have dependent voltages, including Ca2+, Na+, or K+ channels. This is significant as these channels have an important function that can control the progression of epileptic symptoms, including restoring the neuronal membrane to its optimal state.
Additionally, antiseizure medications can also target inhibitory synapses and reduce the excitability of neurones through its mechanism of action on the GABAergic system.
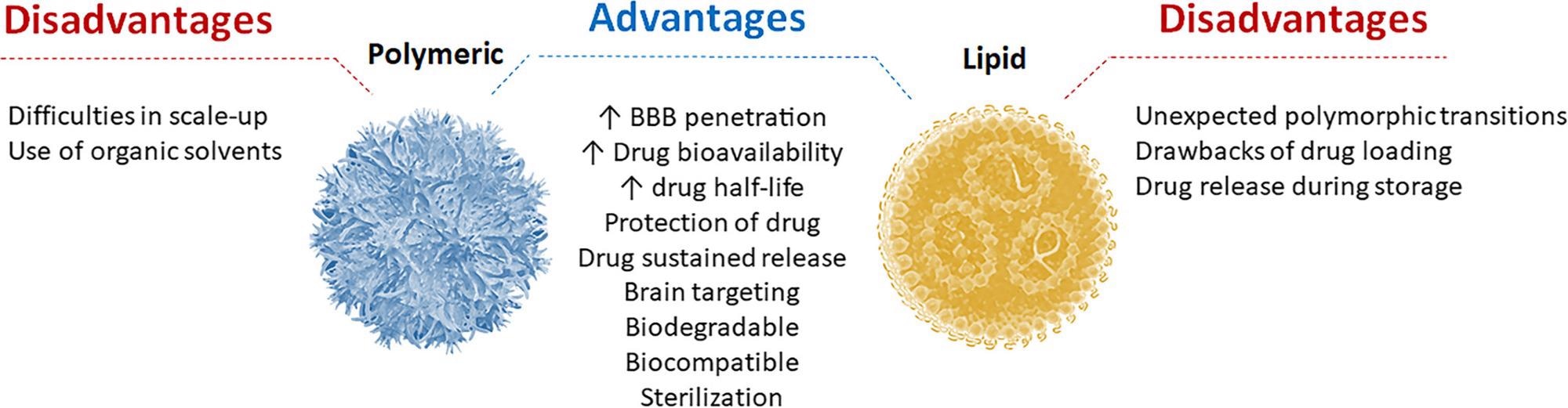
Main advantages and disadvantages of both polymeric and lipid nanoparticles. © Bonilla, L. et al (2021)
However, as previously mentioned, a number of these antiseizure medications result in a lack of response in patients and so room for improvement with nanomedical incorporation could be a potential method of increasing the efficacy of epilepsy treatments.
Biodegradable Nanoparticles for Epilepsy Treatments
The introduction of nanotechnology and nanoparticles for advancing medicine has had significant benefits for patient care, with research being furthered in many different areas.
One such benefit of using nanotechnology consists of nanoparticles, including polymeric nanoparticles (PNP) to cross the blood-brain barrier due to their nanoscale size. This barrier which is usually beneficial for the exchange of nutrients and preventing any damages to the brain microenvironment can be a challenge for conventional drugs that find it difficult to cross due to being too large.
Nanomedicine has allowed the advancement in antiseizure medication in order to cross the barrier as well as increasing the concentration of these drugs in the central nervous system. The use of polymeric nanoparticles can also allow selectivity that can aid in targeting specific receptors through polymeric matrix surface functionalization that can guide the particles to their therapeutic target.
The polymeric matrix material allows the particles to be specialized in order to have specific properties for their desired function and this can be formed through diverse polymers or a combination of different polymers. Polymers that are used most commonly for this purpose include polylactide (PLA), poly(lactide-co-glycolide) (PLGA), chitosan, and polyethylenimine (PEI).
Researchers developing PNPs that encapsulate epilepsy drugs consisting of PLGA nanoparticles have shown results such as reduced dosages with a single dose every 24 hours still having a positive effect on controlling seizures. Additionally, an accumulation of these particles may have even had a neuroprotective effect on the brain.
Other studies have included results of encapsulated antiseizure drugs within PNPs which were 30 times more effective than when used freely without a nanocarrier, with PNPs having a further effect of overcoming drug resistance mediated by P-glycoprotein.
Future Outlook
With the narrow therapeutic margin of antiseizure medication for epilepsy as well as the drug resistance over time, this area of medicine requires further development. The use of polymeric nanoparticles to encapsulate these drugs has resulted in higher efficacy, with seizures being maintained.
The use of these biodegradable particles ensures a lack of toxicity to patients due to being broken down into non-toxic bioproducts while at the target site, which also allows for sustained drug delivery. This safer and more effective alternative of delivering epileptic drugs to patients reduces adverse effects and the need for multiple administrations.
Research for future therapies can target strategies in overcoming permeability limitations within the blood-brain barrier as well as effective drug delivery in the CNS, for which polymeric nanoparticles can be a promising tool. Additionally, the use of nanoparticles for transdermal drug delivery would also be an interesting research point in providing long-term treatment.
However, translation of nanocarriers for clinical use is a difficult challenge that may require governmental regulatory policies to solve. This pharmaceutical and medical process is quite complex and expensive, preventing the advancement of critical patient care. With the use of responsible governmental policies, the development of treatments using nanocarriers for epileptic treatment can truly be advanced.
Continue reading: Progressing Pulmonary Drug Delivery with Nanoparticles.
Reference
Bonilla, L., Esteruelas, G., Ettcheto, M., Espina, M., García, M., Camins, A., Souto, E., Cano, A. and Sánchez‐López, E., (2021) Biodegradable nanoparticles for the treatment of epilepsy: From current advances to future challenges. Epilepsia Open, Available at: https://onlinelibrary.wiley.com/doi/full/10.1002/epi4.12567
Further Reading
Devinsky, O., Vezzani, A., O'Brien, T., Jette, N., Scheffer, I., de Curtis, M. and Perucca, P., (2018) Epilepsy. Nature Reviews Disease Primers, 4(1). Available at: https://doi.org/10.1038/nrdp.2018.24
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.