The awareness of plastic waste contamination and its possible consequences on organisms has drawn a lot of attention. The overwhelming majority of plastics manufactured are disposed of in landfills or the ecosystem. Plastic can shatter into tiny parts known as supplementary plastics once it enters the ecosystem due to mechanical, thermodynamic, as well as other breakdown causes.
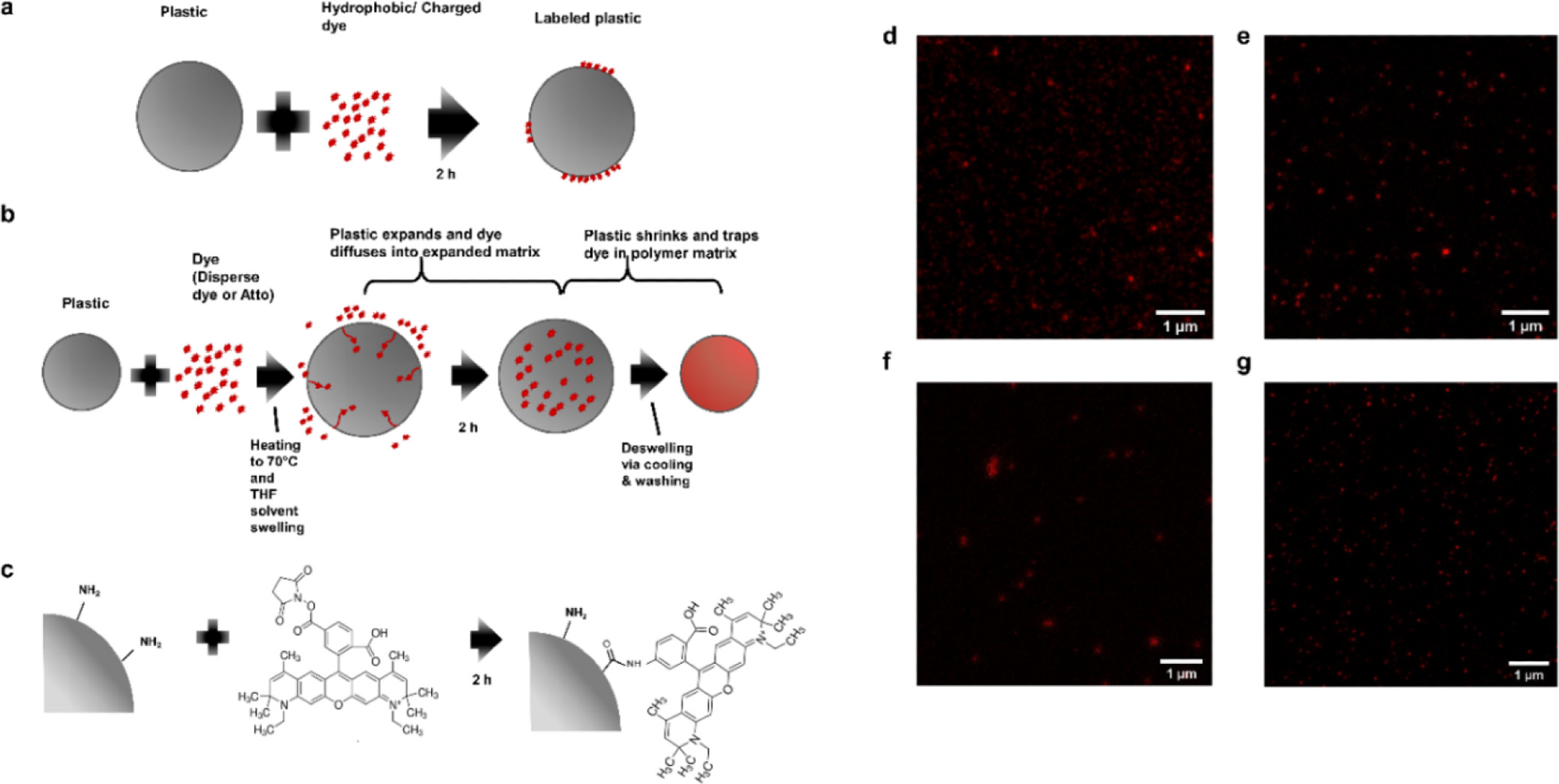
STED-compatible dye labeling techniques (a) passive labeling with Atto 647N where the dye solution and the plastic are simply mixed together; (b) swell labeling with Atto 647N/iDye Poly Blue where the plastic is heated in the dye solution to swell the polymer matrix and allow the dye to enter the polymer matrix (with Atto 647N, THF, a solvent, is added to aid in swelling), after swelling the plastic is cooled and resuspended in DI water to deswell and remove excess dye; (c) covalent labeling by coupling NHS-functionalized Atto 647N to amine groups on functionalized plastic; (d) STED image of passively labeled 100 nm polystyrene beads with Atto 647N; (e) STED image of swell-labeled 100 nm polystyrene beads with Atto 647N; (f) STED image of swell-labeled 100 nm polystyrene beads with iDye Poly Blue; and (g) STED image of covalently labeled 100 nm PS beads with Atto 647N. Image Credit: Nguyen, B et al., Environmental Science Technology
Why Nanoplastics Have Not Been Addressed Effectively
Plastic contamination in the lowest size fractions frequently dominates particulate concentrations in the atmosphere. Despite their prospective ubiquity, little understanding has been established regarding the neurotoxic consequences and processes of the tiniest particulates (i.e., nanoplastics), mostly because they are difficult to visualize in moist substrates such as biomolecules.
A Brief Introduction to Fluorescence Microscopy
Fluorescence microscopy enables accurate and statistical identification of nanoparticles and biomolecules by precise labeling. As a result, fluorescence microscopy has been frequently employed to locate tagged microplastics utilized in environmental investigations in order to track absorption and probable translocation in biological organisms.
Microplastics are often big enough to be identified separately with microscopic observation, enabling the localization of fluorescently labeled nanoplastics even when there is a considerable interference signal from autofluorescence or chemical leakage.
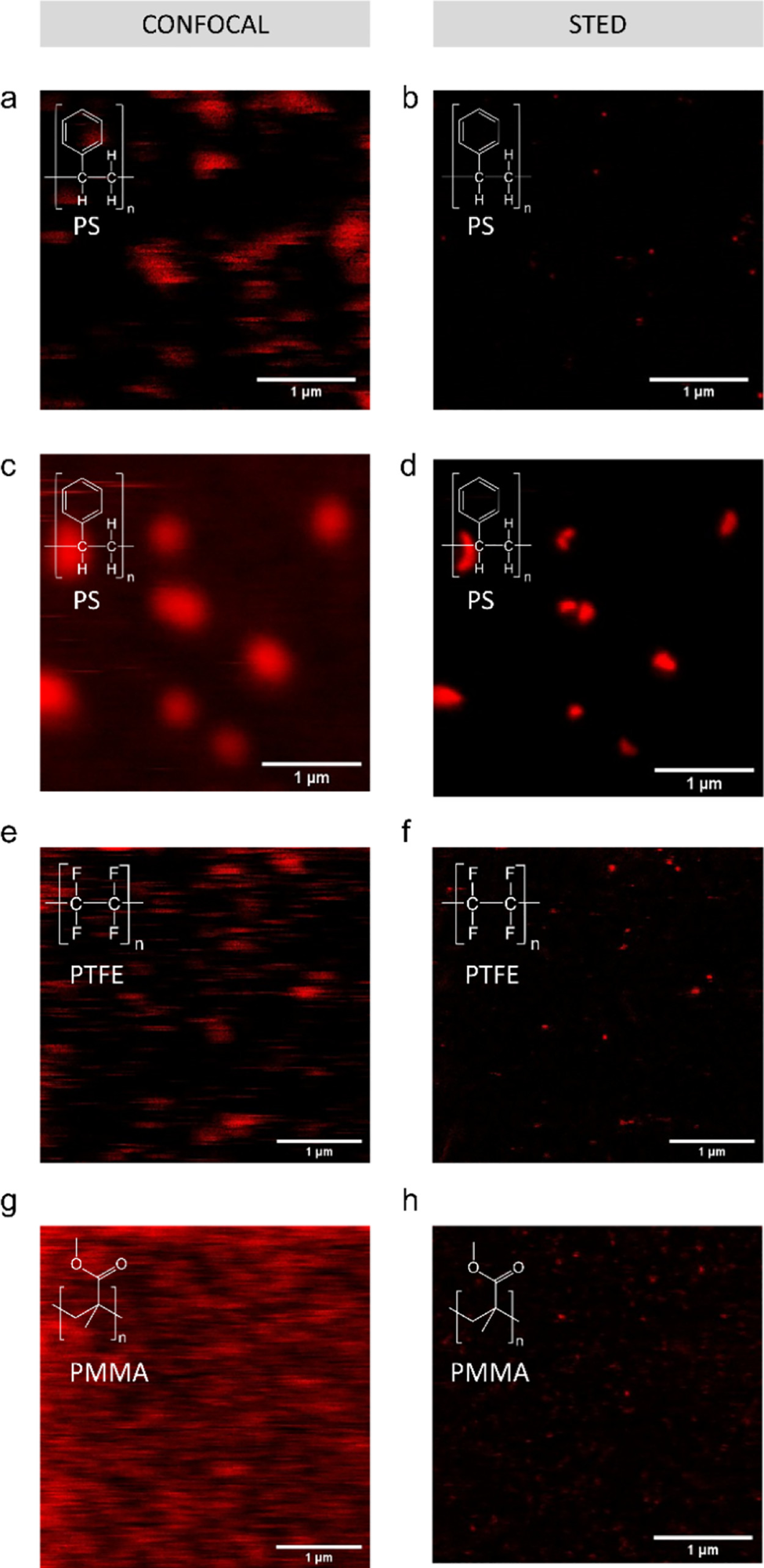
Passive labeling and fluorescent imaging of various nanoplastic types with Atto 647N. Confocal (a) and STED (b) images of debris released from an expanded polystyrene plate exposed to 90 °C in DI water; (c) confocal and (d) STED image of sanding debris from a polystyrene Petri dish; (e) confocal and (f) STED images of PTFE particles; and (g) confocal and (h) STED images of PMMA particles. Confocal and STED images are of the same field of view. Image Credit: Nguyen, B et al., Environmental Science Technology
Limitations of Fluorescence Microscopy for Nanoplastic Imaging
Nano plastics are often too tiny to be identified independently with fluorescence imaging methods presently used in polymer studies, such as laser scanning, optical scanning, and widefield microscopy, based on an optical diffraction limit of 200 nm. As a result, nano plastics are often visible as scattering restricted patches in traditional optical imaging and cannot be properly located in cells or separated from ambient pigment or chemiluminescence.
What Makes Stimulated Emission Depletion (STED) Helpful?
STED imaging accomplishes sub-diffraction precision by concurrently screening material with two laser beams: an activation laser with a spherical focus and an overlaying exhaustion laser with a doughnut-shaped area. The exhaustion lasers inhibit fluorescence emission from the stimulation laser spot's perimeter, significantly limiting the size of the emission area and improving precision beyond the percolation threshold.
However, the pigment suitability of STED imaging is restricted due to the dye's necessity for effective spontaneous emission and excellent stability to minimize extreme photobleaching. Nonetheless, fluorescent compounds suitable for STED imaging are available in multiple hues, including STAR 440 SXP, SeTau 405, and DY-520XL.
Why Spherical Nanoplastics are Employed in Experiments
Since alternative forms of tagged nano plastics are not commercially available, nano plastic treatment investigations use a disproportionate number of round polystyrene nanoscale aggregates. These polystyrene microspheres do not reflect the range of potential ecological microplastic pollution.
Approaches for labeling nano plastics of variable forms and constituents have been devised, and STED imaging has been utilized to scan nano-sized polystyrene rubber with pre-loaded patented fluorescent dyes. However, there are few techniques for florescent labeling nanoplastics of various geometries and polymeric kinds using STED-compatible pigments.
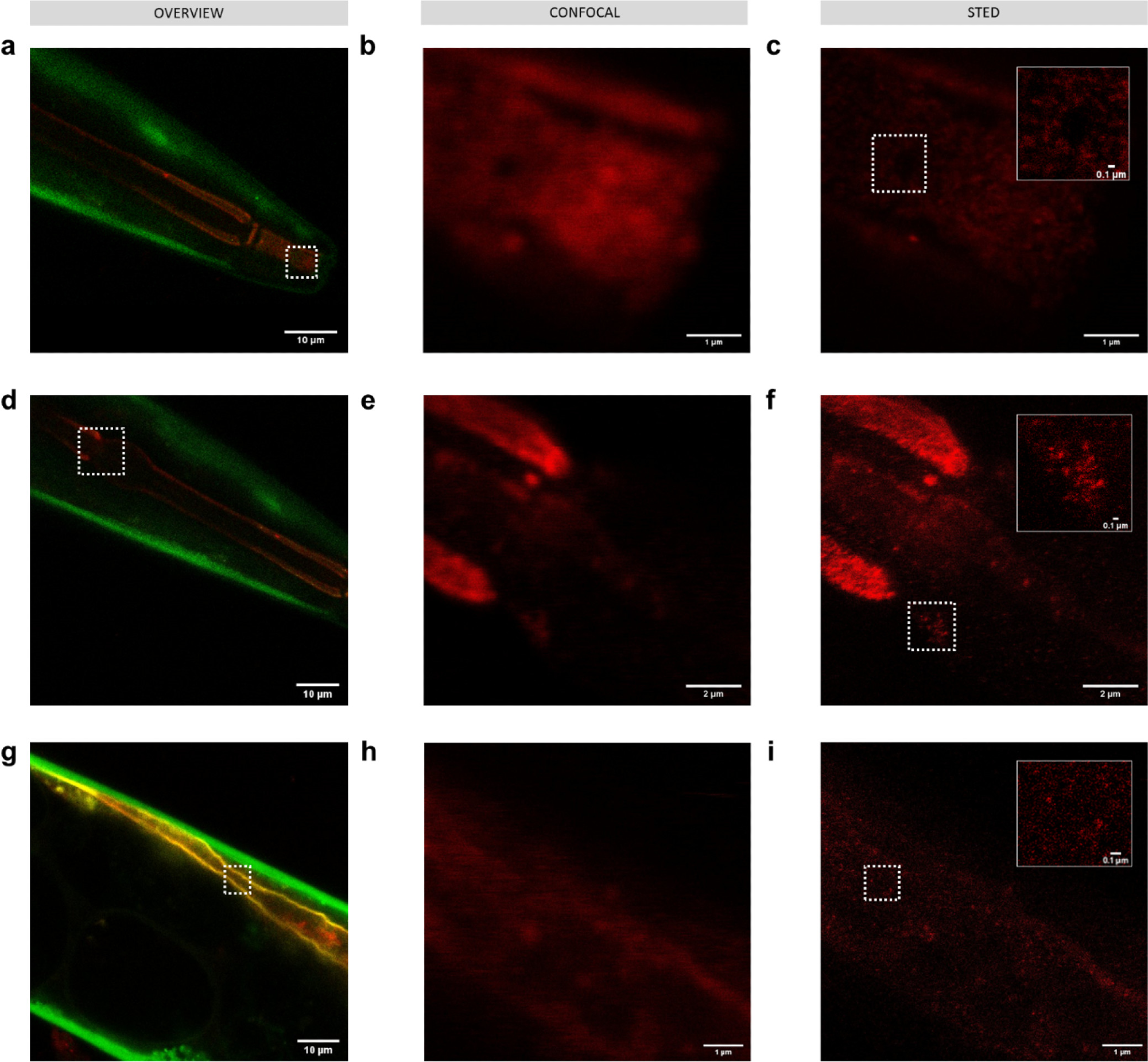
Imaging 50 nm polystyrene nanoplastics passively labeled with Atto 647N (red) in C. elegans KWN117 adult expressing GFP (green) in the body wall and mCherry (yellow) in the apical intestinal membrane. Confocal overview and high resolution of confocal and STED images of the scanned area indicated by white boxes for parts of the digestive track in the mouth (a–c), pharynx (d–f), and intestine (g–i). Insets in STED images correspond to the areas indicated by white boxes. Image Credit: Nguyen, B et al., Environmental Science Technology
Research Findings
STED microscopy with iDye also demonstrated better resolution than traditional laser-scanning fluorescence microscope. When iDye labeling was used, the sharpness was poorer than when Atto 647N labeling was used. This finding was anticipated given that Atto 647N is established to function well with STED imaging, but iDye is an upcycled textile dye rather than a fluorescent nano spherical substance intended specifically for this purpose.
The capacity to observe particles with diffraction-unlimited precision was preserved in nanoparticles identified using the procedures outlined. While the actual average signal strength at all times throughut this test varied from day to day, the nanoplastics were fully evident using STED imaging over the length of the experiment. Interestingly, even polymers weakly tagged with Atto 647N were persistent in oil.
In theory, swell and covalently bonded labeling might give longer labeling durability than passive labeling alone. Fluorescently tagged nanoparticles with nanoscale precision can be used to effectively assess the environmental toxicity of nanostructures other than nanoplastics.
In short, while STED optics have been utilized in the bioengineering framework to investigate the relationships of inorganic nanoparticles with cells in vitro, this study proved the effectiveness of STED spectroscopy in studying nanoparticle interconnections with microbes in potential ecological exposure conditions and with diverse environmental pollutants.
Further Reading
Nguyen, B., & Tufenkji, N. 2022. Single particle-resolution fluorescence microscopy of nanoplastics. Environmental Science Technology. Available at: https://pubs.acs.org/doi/10.1021/acs.est.1c08480
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.