Brown University researchers develop a nanoscale delivery system that improves antifungal treatment effectiveness.
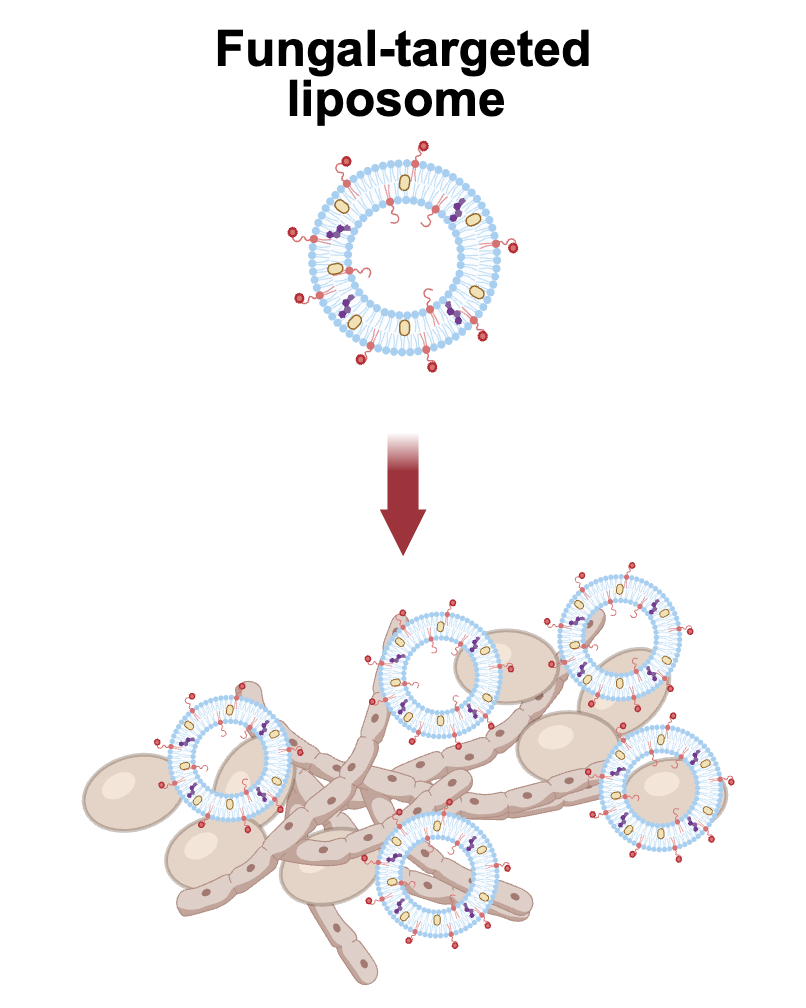 By “decorating” a liposome with a peptide that's naturally attracted to Candida cells, researchers have developed a promising new way to deliver antifungal drugs to fight infections. Image Credit: Shukla Lab / Brown University
By “decorating” a liposome with a peptide that's naturally attracted to Candida cells, researchers have developed a promising new way to deliver antifungal drugs to fight infections. Image Credit: Shukla Lab / Brown University
A new approach from engineers at Brown University could help improve how we treat fungal infections, especially those caused by increasingly drug-resistant Candida species.
In a recent study, the team demonstrated how liposomes - tiny, lipid-based nanoparticle drug carriers - can be engineered to specifically target fungal cells. Their results showed that this method significantly boosted the effectiveness of antifungal drugs, even against tough-to-treat fungal biofilms, without harming healthy human cells.
Fungal infections can be extremely difficult to treat and the drugs at doctors’ disposal are limited. By adding a targeting peptide on the surface of liposomes, we can better target the delivery of an anti-fungal drug to fungal cells, and increase its ability to kill those cells.
Veronica LaMastro, Ph.D. Graduate and Study Lead Author, Biomedical Engineering, Brown University
The team focused their work on Candida auris, an emerging and highly drug-resistant fungus that's become a serious concern in healthcare settings. Between 2017 and 2018, infections from C. auris jumped by more than 300 % in the United States alone.
To better deliver treatments, the researchers turned to liposomes - spherical nanoparticles made from natural and synthetic fats. These particles can carry drugs either inside their core or within their membranes and are often used to stabilize and direct drug therapies. The innovation here was to “decorate” the liposome’s surface with a short chain of amino acids, called a peptide, that naturally seeks out Candida cells. This effectively turns the liposomes into guided delivery vehicles.
After screening multiple peptides, the team found that one, called penetratin, was particularly effective in targeting Candida. They then created liposomes outfitted with penetratin and loaded with posaconazole, an FDA-approved antifungal drug often used to prevent Candida overgrowth.
In lab tests, these customized liposomes showed a much higher likelihood of attaching to Candida cells compared to non-targeted versions. The targeted liposomes also greatly enhanced the antifungal drug’s potency. In some cases, they reduced the amount of drug needed to inhibit fungal growth by up to eightfold - and in preventing biofilm formation, they were effective at doses more than 1,300 times lower than those required for the free drug.
Equally important, the liposomes appeared safe for human cells commonly affected during infections, including those from skin, blood vessels, vaginal tissue, and red blood cells.
To see how this might play out in a real-world infection, the team tested the treatment in a mouse model of intradermal Candida albicans infection. Mice treated with the targeted liposomes showed a 60 % reduction in fungal load compared to those given standard drug-loaded liposomes, an encouraging sign for future clinical applications.
Fungal infections are a vastly understudied area, especially in the engineering and biomaterials communities. But with rising antimicrobial resistance coupled to the increasing use of antifungals in clinical and agricultural settings, this type of work becomes more important. We hope more researchers will recognize that and do more work in this field.
Anita Shukla, Professor, School of Engineering, Brown University
The team plans to expand on this work by testing their delivery system with drugs used to treat, not just prevent, established fungal infections.
The study, published in Advanced Functional Materials, was supported by the National Science Foundation (CBET-1942418). Co-authors include Dominique Walker, Joanne Liu, and Tobias Meng-Saccoccio.
Journal Reference:
LaMastro, V., et al. (2025) Peptide-Decorated Liposomes Enhance Fungal Targeting and Antifungal Drug Delivery. Advanced Functional Materials. doi.org/10.1002/adfm.202508570