Feb 27 2008
Indiana University Bloomington chemists have designed an organic molecule that binds negatively charged ions, a feat they hope will lead to the development of a whole new molecular toolbox for biologists, chemists and medical researchers who want to remove chlorine, fluorine and other negatively charged ions from their solutions.
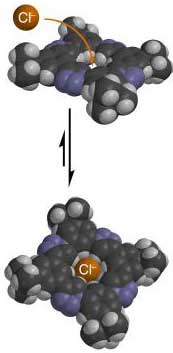 This complex organic molecule is capable of binding a negatively charged ion (chloride). The versatile, easy-to-make molecule may represent a new family of binding agents for use in biology and medicine
This complex organic molecule is capable of binding a negatively charged ion (chloride). The versatile, easy-to-make molecule may represent a new family of binding agents for use in biology and medicine
"What we've done is create an efficient synthesis that gives us access to a whole new family of binding agents," said Amar Flood, who reports the discovery with postdoctoral scholar Yongjun Li in Angewandte Chemie this week. "The synthesis is extremely modular, as well, so we imagine these molecules can be easily modified to bind a wide variety of negative ions with great specificity."
Chelating agents are small molecules that grab atoms (or, sometimes, even smaller molecules) out of a solution and hold onto them. Chelators play a valuable role in both nature and laboratory settings. For example, the human protein calmodulin not only grabs positively charged calcium ions out of the solution surrounding it, it also influences cell processes according to how many calcium ions it has grabbed. In labs, EDTA (ethylenediaminetetraacetic acid) is frequently used to remove calcium and magnesium ions so that chemical reactions go faster or more efficiently.
Many organic molecules exist that can bind positively charged ions, or cations, and this has much to do with the fact that it is easy to synthesize organic molecules with negatively charged parts. It is those negatively charged parts that interact with positive ions, or cations, grabbing them out of solution and holding onto them so the cations cannot react or interfere with other processes.
Attempts at manufacturing organic binding agents with positively charged parts is not hard, but designing them in such a way that they don't attract the attention of solvent molecules has been a major challenge for chemists.
Flood and Li's solution was to create a donut-shaped organic molecule whose center would serve as the binding spot. A halide ion might fit snugly inside the hole, but the arrangement of atoms surrounding the hole would exclude any solutions.
Flood also wanted the synthesis of such a molecule to be cheap, easy and flexible, so he looked to the "click chemistry" devised by Scripps Research Institute chemist K. Barry Sharpless. Click chemistry is an efficient method of joining molecules together to form larger molecules. Flood's particular application of click chemistry results in an eight-member macrocycle with a 3.7 angstrom hole in the middle. The more-or-less symmetrical molecule that Flood and Li built contains four triazole rings sporting three nitrogen atoms each. It is presumed the nitrogens withdraw electron density from the carbon and hydrogen atoms closest to the molecule's hole, thereby creating an alluring binding spot for fluorine or chlorine ions. This binding is made all the more orderly because the macrocycle is preorganized to host its anionic guest.
"This thing is so easy to make," Flood said. "The triazole moiety has got more character to it than meets the eye. It's not just a byproduct of the click chemistry. We see lots of potential in it."
The other four members of Flood and Li's eight-member ring are entirely substitutable. Modifying these may give the chelator different binding affinities for a given anion.