|
Magnetic fluids with narrow size distributions exhibit useful properties for a number of technical and biomedical applications. The magnetic properties of magnetic fluids depend strongly on the size of the particles and the concentration of the magnetic material in dispersion. The well known magnetite magnetic fluids have a good stability. However, the magnetic properties of these materials are not sufficient for a number of purposes. Consequently, stable magnetic fluids on the basis of nano-sized metallic Fe-, Co- or Fe/Co alloy colloids are very interesting materials.
Preparation of Colloidal Magnetic Particles by Thermolysis of Metal Carbonyls
Air-stable, colloidal, metallic particles (Fe, Co, Ni, and Fe/Co) with a narrow size distribution are accessible via the thermolysis of metal carbonyls in the presence of aluminum alkyls. Subsequent “Smooth Air Oxidation” leads to long term stable metallic magnetic nanoparticles, as was evidenced by XANES and other physical methods.
Magnetic Fluids Prepared by Peptisation or Metallic Nanoparticles
The isolated particles can be dried for powders or peptised with the help of surfactants resulting in remarkably stable metallic magnetic fluids (MF) applicable for a number of practical purposes. The influence of the aluminum alkyls during the decomposition of Co2(CO)8 in toluene was studied by IR spectroscopy. It was found that the aluminum alkyls act as catalysts for the thermal decomposition of the metal carbonyl via Co4(CO)12 and higher Co carbonyls to give “monodisperse” Co nanoparticles.
Carrier Liquids for Magnetic Fluids
Via peptisation of the obtained metallic or bimetallic magnetic nanoparticles, magnetic fluids in different carrier liquids, such as water, hydrocarbons, kerosene, mineral and vacuum oils, and silicones, are obtained by using suitable surfactants for each carrier liquid. Suitable surfactants combine strong adsorption properties on the particle surface, good protecting abilities to prevent the particles from oxidation, and good solubility in carrier liquids. The optimal quantity of the surfactants and the best conditions of stabilization have to be elaborated individually.
Water Based Magnetic Fluids
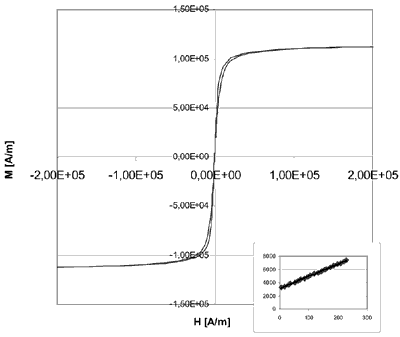
Water based magnetic fluids are also accessible via mono-, bi- or polylayers formed around the particles when ionic, non-ionic, or double surfactants are applied. These air stable magnetic fluids have a high potential for a number of technical and biomedical applications. The magnetic fluids were investigated for their magnetic properties by TEM (Transmission Electron Microscopy), by Moessbauer spectroscopy, by DRIFTS (Diffuse Reflection Infrared Fourier Transform Spectroscoy), UPS (Ultraviolet Photoelectron Spectroscopy), and MIES (Metastable Impact Electron Spectroscopy). The resulting magnetic fluids exhibit extraordinary magnetic properties at low concentrations of the magnetic material (see Fig. 1) and show unusual high magneto-viscous effects.
|
|
|
Figure 1. High saturation magnetization (120.1 kA/m = 151mT = 1510G) of a 8.21 Vol-% Co-Kerosene fluid (particle size 11nm).
|
Properties of Magnetic Fluids after Peptisation
By TEM it was confirmed that the particle size was not altered during the peptisation process. Moessbauer investigations of Fe and Fe/Co magnetic fluids in kerosene revealed that the particles consist of a metallic or bimetallic core which is protected against air-oxidation by a shell of Fe3+ ions (probably Fe carboxylates and/or Fe oxides).
The resulting Moessbauer spectra show a superimposition of the spectra of Fe or the Fe/Co alloy (major component) and Fe3+ as the minor component. In the case of the Co(0) fluid, according to DRIFTS, UPS, and MIES, the anti-corrosive shell consists of Co carboxylates and Co oxides.
A complete list of references can be found by referring to the original document.
|