The article details the development of a new bioactive composite resin containing silver/zinc oxide (Ag/ZnO) nanoparticles, with orthodontic applications. Here, its mechanical, biocompatible, cytotoxic, and antimicrobial properties are discussed.
Image Credit: Prostock-studio/Shutterstock.com
Orthodontic treatment harbors many advantages, but it also poses complications like demineralization and tooth discoloration around brackets and the bonded areas in the form of white spot lesions (WSL). Patients obtaining a complete treatment plan are more vulnerable to caries (decay) and demonstrate a substantial increase in Streptococcus mutans counts in their plaque and saliva.
This can be avoided by using fluoride-containing materials, particularly fluoride varnishes. However, these possess two major disadvantages—regular patient cooperation and a moderate effect on preventing WSL and caries. Nanoparticles (NPs) are broadly used for their antimicrobial effects and earlier research has demonstrated that ZnO nanoparticles are safe and biocompatible to use in dental and medical applications.
Silver NPs also demonstrated biocompatible behavior. Ag/ZnO (AZ) nanoparticles are white, and when introduced into composite resins of the same color do not lead to esthetic problems. Also, combining nanoparticles with both silver and zinc oxide increases their antibacterial properties.
This article evaluates the impact of introducing ZnO and AZ nanoparticles into orthodontic composite resins on the antibacterial properties, biocompatibility, and shear bond strength of these composite resins. This research was published in the journal Evidence-Based Complementary and Alternative Medicine.
Methodology
Preparation of Nanoparticles
Zinc acetylacetonate, sodium hydroxide, AgNO3, ethanol (absolute), and starch were used to synthesize ZnO and Ag/ZnO nanoparticles and are named Z and AZ.
Nanoparticles were mixed continuously with no-mix self-cure composite resin, and highly polished samples with similar surface roughness (Ra) values were obtained. X-ray diffraction technique characterized the crystalline structure of Ag-doped ZnO nanoparticles.
Shear Bond Strength (SBS) Test
Various resin composites were developed in four separate experimental groups—Group 1 with composite resin for bonding of brackets without nanoparticles (O), groups 2–4, had composite resin with ZnO nanoparticles (Z), composite resin with ZnO nanoparticles, and silver ions (A&Z), and composite resin with Ag/ZnO nanoparticles (AZ) respectively.
The surface of teeth was cleaned with fluoride-free pumice paste and washed for 10 seconds by running water followed by etching. Here, a thin layer of autopolymerization adhesive was applied on an etched section of teeth and bracket base.
The SBS test was carried out by placing the blocks in the Hounsfield Test Equipment.
Zn and Ag Release
Nanoparticle-containing composite discs were developed and stored in a dry place and were immersed individually in artificial saliva for 30 days, replacing the immersing solution every seven days. The solutions were later analyzed by inductively coupled plasma optical emission spectrometry to determine Zn and Ag ionic concentrations released from the composite.
Antibacterial Activity Assay
The antimicrobial activity of the components was analyzed with the micro broth dilution (MIC) method. S. mutans ATCC 35668, Staphylococcus aureus ATCC 25923, Lactobacillus gasseri ATCC 33323, Escherichia coli ATCC 25922, and Candida albicans ATCC 10231, the common pathogens were selected and cultured in Mueller-Hinton agar overnight at 37 °C.
Yeasts were cultured in Mueller-Hinton agar plus 1% glucose under aerobic conditions.
Composite resins of various concentrations were exposed with pathogens along with positive and negative controls.
MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium Bromide) Assay for Cell Viability
HGF (human gingival fibroblast) cells were incubated in 96-well plates and incubated for 24 hours at 37 °C and 5% CO2. The cells were then grouped into four triplicates, blank, Z, AZ, and A&Z nanoparticles, and were treated. After incubation, the absorbance was read with an ELISA plate reader at 570 nm wavelength.
Wettability Measurements
The wettability of the samples was analyzed by measuring the contact angles (the angle at which the liquid interface met the solid surface of the composite disc) of distilled water on composite resins with Adobe Photoshop® software. The contact angle of the surface of the drop was measured 20 seconds after stabilization of the droplet.
Statistical Analysis
Data analyses were carried out with Anderson–Darling and Levine test to assess the homoscedasticity and normality. Standard deviations were calculated along with a one-way ANOVA test and Tukey’s test.
Results
Characteristics Analysis
The powder XRD patterns of the prepared ZnO and Ag/ZnO is depicted in Figure 1. No characteristic peaks of impurity phases like Zn, Zn(OH)2, Ag, or Ag(OH) were observed.
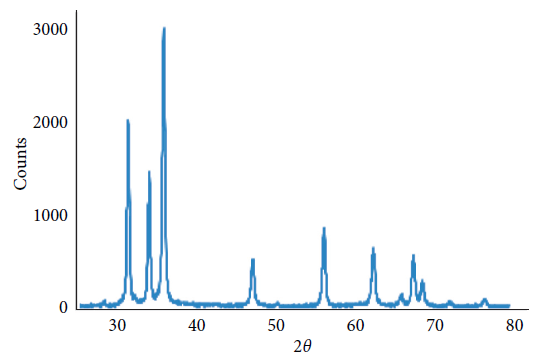
Figure 1. XRD pattern of Ag/ZnO nanoparticles. The existence of high-grade peaks proves the creation of a ZnO system. Image Credit: Kachoei, et al., 2021.
The distribution of nanoparticles in composite resins is shown in Figure 2.
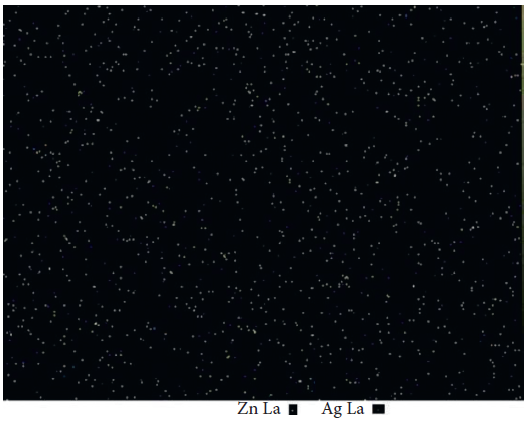
Figure 2. Zn and Ag map of the resin composite containing 10 wt% Ag/ZnO. Light yellow spots represent Zn, and blue ones represent Ag element. Image Credit: Kachoei, et al., 2021.
After a period of 30 days, composites comprising AZ, A&Z, Z nanoparticles displayed no substantial release of silver or zinc ions.
Shear Bond Strength Test
Four kinds of orthodontic composite resins were evaluated. Table 1 depicts the data of shear bond strength tests in each study group separately.
Table 1. The descriptive data of shear bond strength in each study group. Source: Kachoei, et al., 2021.
| Group |
Number |
Mean of shear
bond strength
(MPa) |
Std.
deviation |
Minimum |
Maximum |
| A (O) |
30 |
19.03 |
4.12 |
13.42 |
28.95 |
| B (Z) |
30 |
16.35 |
1.11 |
7.90 |
32.63 |
| C (A&Z) |
30 |
13.61 |
0.73 |
8.64 |
22.61 |
| D (AZ) |
30 |
20.49 |
1.03 |
10.48 |
32.17 |
| Total |
120 |
17.37 |
0.51 |
7.90 |
32.63 |
It was seen that the mean shear bond strength value in the A&Z group was lesser than that in other groups (Figure 3).
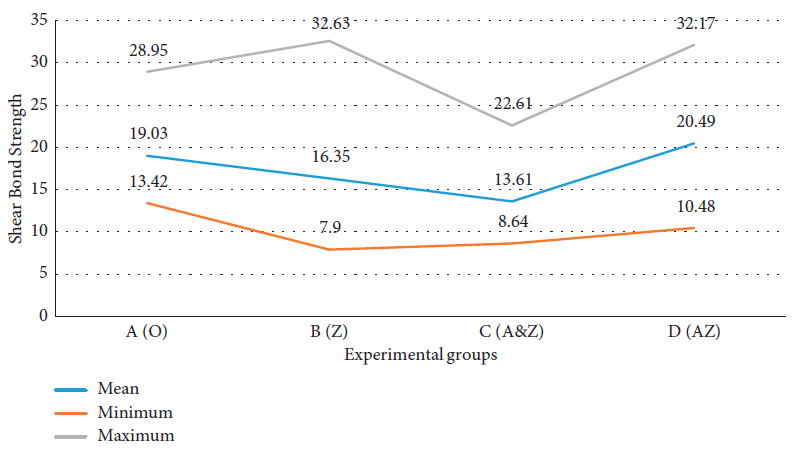
Figure 3. Comparison of shear bond strength in each study group. Image Credit: Kachoei, et al., 2021.
Antimicrobial Properties
Table 2 reveals the antimicrobial properties and is found to exhibit slight antimicrobial properties against Gram-negative (E. coli) pathogen and ZnO with 10% concentration depicted greater antimicrobial properties than 5% concentration.
Table 2. Antimicrobial effects of the test nanoparticles. Source: Kachoei, et al., 2021.
Test groups
containing Ag
and ZnO (wt %) |
Bacteria group (colony-forming unit) |
S. mutans
ATCC
35668 |
S. aureus
ATCC
25923 |
E. coli
ATCC
25922 |
C. albicans
ATCC
10231 |
Lactobacillus
gasseri |
| A.ZnO [10%] |
0 |
0 |
0 |
1500 |
0 |
| B.ZnO [5%] |
0 |
0 |
500 |
50000 |
0 |
| C.Ag [0.1%] /ZnO [10%] |
0 |
0 |
0 |
10000 |
0 |
| D.Ag [0.05%] /ZnO [5%] |
0 |
0 |
0 |
20000 |
0 |
| E.Ag [0.1%] /ZnO [5%] |
0 |
0 |
7 |
50000 |
0 |
| F.Ag [0.05%] /ZnO [5%] |
0 |
0 |
32 |
>10000 |
0 |
| Control (−) |
0 |
0 |
0 |
0 |
0 |
| Control (+) |
>10000 |
>10000 |
>10000 |
>10000 |
>10000 |
Viability Test
MTT test results revealed the cytotoxicity of AZ and ZnO nanoparticles. Figure 4 depicts the data.
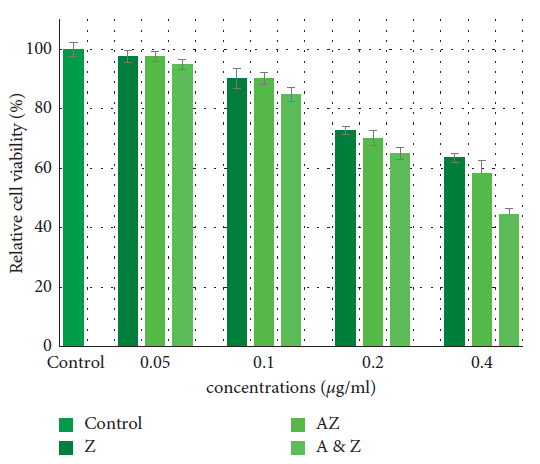
Figure 4. Effects of the Ag/ZnO and ZnO nanoparticles on cell viability in HGF cells. Data are expressed as the mean of percent cell viability compared to control after exposure for 24 hours ± standard deviation (n = 3) ( = 0.05). Image Credit: Kachoei, et al., 2021.
Wettability Measurements
Table 3 details the means and standard deviations of the contact angles of the analyzed groups. The contact angles of all groups were less than 90°, and both of them are hydrophilic.
Table 3. The contact angles of the composites in degrees (mean ± SD). Source: Kachoei, et al., 2021.
| Composite |
Contact angle |
| Conventional composite resin (O) |
48.40 ± 2.24 |
| ZnO nanoparticles composite resin (Z) |
51.08 ± 1.86 |
| ZnO nanoparticles and silver ions composite resin (A&Z) |
54.32 ± 3.54 |
| Ag/ZnO nanoparticles composite resin (AZ) |
55.74 ± 5.38 |
Discussion
Oral hygiene during orthodontic treatment is always challenging due to patient cooperation. Methods that avoid this are therefore preferable. This article evaluates the effect of incorporating nanoparticles into orthodontic composite resins.
Among the nanoparticles, AZ nanoparticles showed antibacterial activity even at lower concentrations. ZnO and ZnO nanoparticles having Ag silver ions showed antibacterial activity at greater concentrations.
The results showed strong antimicrobial properties for these nanocomposites, indicating that these composites had significant antimicrobial properties against S. mutans, S. aureus, and L. gasseri but less power against E. coli and no effect against C. albicans.
The nanocomposites can maintain their properties for a long time without delaying the nanoparticles. Also, the analyzed nanocomposites are hydrophobic, and the absorption of bacterial plaques also decreased, which is a significant advantage in orthodontic use.
Conclusion
The current research employed different nanoparticles (ZnO, ZnO and silver ions, and Ag/ZnO synthesized) into orthodontic composite resins and found that they had antibacterial properties against oral pathogens. The introduction of ZnO nanoparticles with silver ions reduced the shear bond strength, but there were no changes in mechanical properties.
Continue reading: Improving Dental Restorative Materials Using Nanotechnology.
Journal Reference:
Kachoei, M., Divband, B., Rahbar, M., Esmaeilzadeh, M., Ghanizadeh, M., Alam, M. (2021) A Novel Developed Bioactive Composite Resin Containing Silver/Zinc Oxide (Ag/ZnO) Nanoparticles as an Antimicrobial Material against Streptococcus mutans, Lactobacillus, and Candida albicans. Evidence-Based Complementary and Alternative Medicine. Available at: https://doi.org/10.1155/2021/4743411.
References and Further Reading
- Dastjerdi, E. V., et al. (2018) Bond strength of an orthodontic adhesive containing an antibiofilm agent (octafluoropentyl methacrylate). Contemporary Clinical Dentistry, 9, pp. S39–S44. doi.org/10.4103/ccd.ccd_2_18.
- Sodagar, A., et al. (2016) Evaluation of the antibacterial activity of a conventional orthodontic composite containing silver/hydroxyapatite nanoparticles. Progress in Orthodontics, 17(1), p. 40. doi.org/10.1186/s40510-016-0153-x.
- Moghadam, N. C. Z., et al. (2018) Effects of laser and fluoride on the prevention of enamel demineralization: an in vitro study. Journal of Lasers in Medical Sciences, 9(3), pp. 177–182. doi.org/10.15171%2Fjlms.2018.32.
- Salmerón-Valdés, E. N., et al. (2016) Tooth demineralization and associated factors in patients on fixed orthodontic treatment. Scientific Reports, 6(1), p. 36383. doi.org/10.1038/srep36383.
- Hemmati, M. A., et al. (2017) Evaluating the physical properties of novel zinc phosphate and zinc polycarboxylate cements containing zinc oxide nanoparticles. Avicenna Journal of Dental Research, 9(3), p. e60720. doi.org/10.5812/ajdr.60720.
- Weir, E., et al. (2008) The use of nanoparticles in antimicrobial materials and their characterization. The Analyst, 133(7), pp. 835–845. doi.org/10.1039/b715532h.
- Borzabadi-Farahani, A., et al. (2014) Nanoparticles in orthodontics, a review of antimicrobial and anti-caries applications. Acta Odontologica Scandinavica, 72(6), pp. 413–417. doi.org/10.3109/00016357.2013.859728.
- Hadadi, A., et al. (2014) Diabetic foot: infections and outcomes in Iranian admitted patients, Jundishapur. Journal of Microbiology, 7(7), p. e11680. doi.org/10.5812%2Fjjm.11680.
- Hojati, S. T., et al. (2013) Antibacterial, physical and mechanical properties of flowable resin composites containing zinc oxide nanoparticles. Dental Materials, 29(5), pp. 495–505. doi.org/10.1016/j.dental.2013.03.011.
- Ahn, S.-J., et al. (2009) Experimental antimicrobial orthodontic adhesives using nanofillers and silver nanoparticles. Dental Materials, 25(2), pp. 206–213, doi.org/10.1016/j.dental.2008.06.002.
- Shahi, S., et al. (2019) A review on potential toxicity of dental material and screening their biocompatibility. Toxicology Mechanisms and Methods, 29(5), pp. 368–377. doi.org/10.1080/15376516.2019.1566424.
- Kachoei, M., et al. (2016) Zinc-oxide nanocoating for improvement of the antibacterial and frictional behavior of nickel-titanium alloy. Nanomedicine, 11(19), pp. 2511–2527. doi.org/10.2217/nnm-2016-0171.
- Mehdipour, M., et al. (2016) A comparison of the effect of triamcinolone ointment and mouthwash with or without zinc on the healing process of aphthous stomatitis lesions. Journal of Dental Research, Dental Clinics, Dental Prospects, 10(2), pp. 87–91. doi.org/10.15171/joddd.2016.014.
- Tavassoli-Hojjati, S., et al. (2012) Evaluation of the effect of fluoride gel and varnish on the demineralization resistance of enamel: an in vitro. Journal of Islamic Dental Association of IRAN, 24(2), pp. 28–34.
- Garmasheva, I., et al. (2016) Lactobacillus species mediated synthesis of silver nanoparticles and their antibacterial activity against opportunistic pathogens. In Vitro Bioimpacts, 6(4), pp. 219–223. /doi.org/10.15171/bi.2016.29.
- Alt, V., et al. (2004) An in vitro assessment of the antibacterial properties and cytotoxicity of nanoparticulate silver bone cement. Biomaterials, 25(18), pp. 438–439. doi.org/10.1016/j.biomaterials.2003.10.078.
- Poosti, M., et al. (2013) Shear bond strength and antibacterial effects of orthodontic composite containing TiO2 nanoparticles. The European Journal of Orthodontics, 35(5), pp. 676–679. doi.org/10.1093/ejo/cjs073.
- Eslamian, L., et al. (2020) Evaluation of the shear bond strength and antibacterial activity of orthodontic adhesive containing silver nanoparticle, an in-vitro study. Nanomaterials, 10(8), p. 1466. doi.org/10.3390/nano10081466.
- Guiraldo, R. D., et al. (2016) Evaluation of shear strength of brackets with different dental composites and enamel roughness, Applied Adhesion Science, 4(1), p. 8. doi.org/10.1186/s40563-016-0065-5.
- Garcia-Contreras, R., et al. (2015) Mechanical, antibacterial and bond strength properties of nano-titanium-enriched glass ionomer cement. Journal of Applied Oral Science, 23(3), pp. 321–328. doi.org/10.1590%2F1678-775720140496.
- Sodagar, A., et al. (2017) Effect of TiO2 nanoparticles incorporation on antibacterial properties and shear bond strength of dental composite used in Orthodontics. Dental Press Journal of Orthodontics, 22(5), pp. 67–74. doi.org/10.1590/2177-6709.22.5.067-074.oar.
- Argueta-Figueroa, L., et al. (2015) An evaluation of the antibacterial properties and shear bond strength of copper nanoparticles as a nanofiller in orthodontic adhesive. Australian Orthodontic Journal.