Lipid-based nanoparticles have gained tremendous attention within the scientific community due to the wide range of applications in biomedical research. This has included use as a component within COVID-19 vaccines as well as within liposomes for drug formulation. These valuable nanoparticles can be modified using various strategies for their associated applications, which will be further explored in this article.
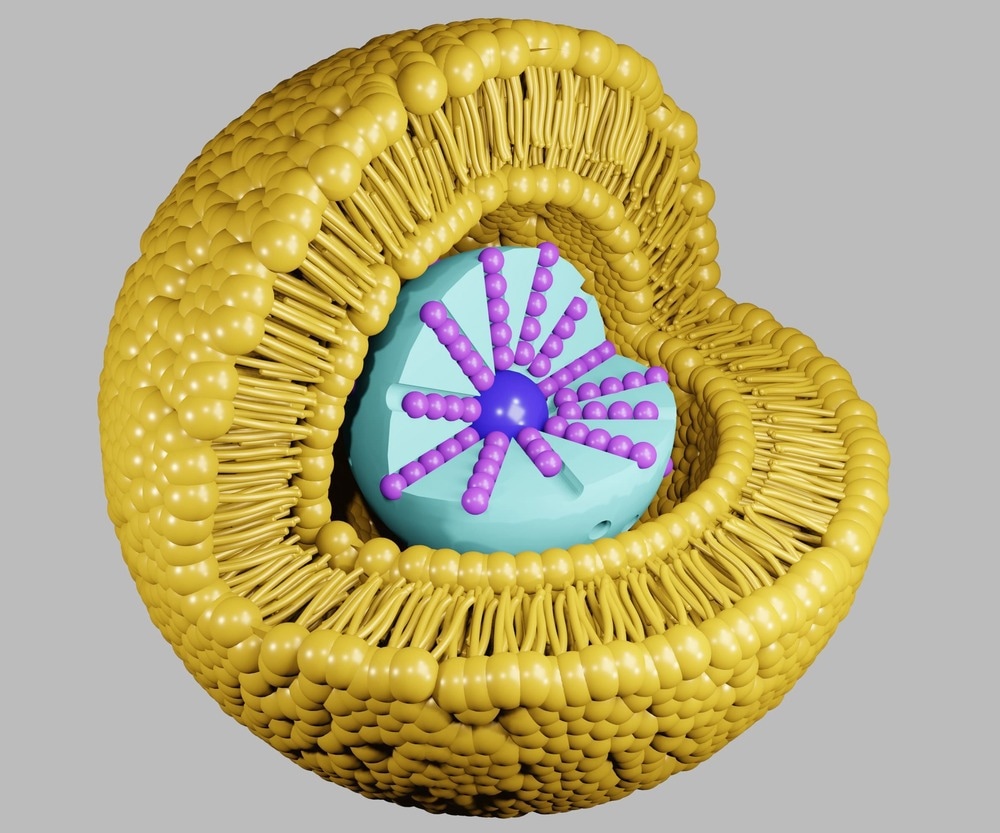
Image Credit: Love Employee/Shutterstock.com
Lipid-based nanoparticles have been invaluable within nanomedicine for various applications, including drug delivery. These nanoscale components have been developed and have been commercialized to increase the efficacy as well as safety of available drugs.
An example of this includes Doxil, an approved FDA nanomedicine product comprising liposomes, which aimed to improve the safety of a previously developed drug named doxorubicin.
These particles have also played a significant role in the development of mRNA vaccines for the COVID-19 virus, with benefits including protection of the mRNA that was encapsulated, as well as increasing intracellular delivery.
Other benefits of lipid-based nanoparticles include biodegradability, low immunogenicity, incorporation of lipophilic drugs, and advantages consistent with the nanoscale (1-100 nm), such as high surface area to volume ratio and surface functionalization.
Lipid-Based Nanoparticle Modifications for Biomedicine
While nanotechnology has been very useful for the advancement of medicine, there have been many strategies researchers have experimented with to develop these particles for an increased desired effect.
These developments have included improving the interactions these particles have with mucus, the targetability of cells, crossing barriers, as well as increasing the efficacy of drug delivery release.
Improving Mucus Interactions
Mucus is secreted by goblet cells and can be described as a complex hydrogel that is based on a biopolymer found on epithelial mucosae, including eyes, airways, and the gastrointestinal tract. The main function of this hydrogel is to cover the epithelial membrane to lubricate and maintain hydration, ensuring oxygen and waste products such as carbon dioxide can be effectively exchanged.
This component holds significance within the body, with mucus comprising proteins called mucins that predominantly make up the structure of mucus and provide known properties of mucus such as adhesion, cohesion, and a gel-like attribute.
Mucins are significant in medicine and biomedical research as dysfunction within these proteins, including an overexpression or reduced expression can result in diseases and disorders such as ulcerative colitis, dry eyes, and even cancer.
The use of nanomedicine to target mucus and mucus-based areas such as the gastrointestinal tract has included the development of lipid formulations with both specific and non-specific targeting.
The modification of lipid-based nanoparticles for targeting this component has commonly involved using chitosan. The benefits of this include evidence demonstrating nanoparticles that have remained in their target mucosal area of concern for a longer period, ensuring a higher availability level of the drug.
Lipid-based nanoparticles that target mucus aim to increase drug absorption at a systemic level within oral and pulmonary routes. They have also been developed to improve mucus penetration and ensure effective drug delivery to the tissue underneath. An effective strategy for this objective has included densely coating the surface of drug nanocarriers with PEG, as well as other polymers such as poly(vinyl alcohol), poly(2-alkyl-2-oxazoline), and poly-(N-(2-hydroxypropyl)methacrylamide).
The development of lipid-based nanoparticles that undergo modifications to enhance their properties for biomedical applications is significant for improving human health and addressing mucus-related disorders such as cystic fibrosis and inflammatory bowel disease.
Enhancing Targetability
The property of precise targeting that has been associated with nanotechnology and nanomedicine has been a revolutionary concept for healthcare and biomedical research. This notion has enabled researchers to investigate how nanoscale particles can be exploited for targeted therapeutics and drug delivery to enhance the level of care provided to patients.
This is significant for therapeutics as specific targeting involves the recognition of cell targets, followed by selective binding and drug delivery to an area of concern.
The benefits of targeting drugs encapsulated by nanocarriers to targeted areas include reducing systemic toxicity associated with the drug, which ensures healthy tissue is protected from exposure. This would be especially beneficial for chemotherapy drugs that carry a high level of toxicity for eradicating cancerous cells; however, they also damage healthy cells, which can be limited via specific targeting nanoparticles.
Lipid-based nanoparticles have been modified to improve the property of targetability through surface functionalization. Here, ligands are attached to the surface of nanoparticles to enable the recognition of target molecules.
The types of ligands that can be used to ensure specific targeting of lipid-based nanoparticles include an array of biological components, comprising antibodies that can precisely target antigens, as well as proteins and aptamers.
The choice of ligand depends on the targeted cell type, as aptamers, which are short single-stranded sequences of nucleic acids can be used for a higher level of selectivity and specificity for their antigen compared to antibodies. Aptamers may also be preferred due to being nonimmunogenic in nature and having stability when in higher temperatures, unlike antibodies which may denature.
Another example of a polymeric ligand is hyaluronic acid, which can target the CD44 receptor; this is significant as the CD44 receptor is overexpressed within various cancer types such as ovarian cancer. The use of using a ligand that targets this critical receptor can hold great potential for the treatment of ovarian cancer.
Challenges and Translation Significance
The use of nanomedicine has provided innovative solutions for many researchers within medicine, including cancer therapy; however, there are many challenges nanomedicine products face, preventing clinical translation.
While modified lipid-based nanoparticles, including a PEGylated surface, have become available on the market, the progression of cell-specific targeted nanomedicine products has been stunted. This may be due to having inefficient penetration by targeted liposomes at the target site; however, a meta-analysis has demonstrated active targeting may increase the accumulation of these particles within tumor sites, with comparison to passive targeting showing values from 0.6% to 0.9%.
A 2021 study also reported most chemoimmunotherapy nanomedicine products as still being in preclinical and clinical trials, with clinical translation being limited due to obstacles such as complexity in nanoparticle design that prevent large-scale production. Other obstacles can also include researching the safety of novel materials incorporated into the nanoparticles as well as controlling drug release volume and ratio of drugs for combination therapies.
Addressing these key obstacles can improve the clinical translation of lipid-based nanoparticles that have been modified to enhance the efficacy of therapeutics. These modifications are a significant step for innovating medicine for precise treatment management for diseases and disorders with a high mortality rate and no cure, such as cancer.
References and Further Reading
García-Pinel B, Porras-Alcalá C, Ortega-Rodríguez A et al. Lipid-Based Nanoparticles: Application and Recent Advances in Cancer Treatment. Nanomaterials. 2019;9(4):638. doi:10.3390/nano9040638
Wang T, Suita Y, Miriyala S, Dean J, Tapinos N, Shen J. Advances in Lipid-Based Nanoparticles for Cancer Chemoimmunotherapy. Pharmaceutics. 2021;13(4):520. doi:10.3390/pharmaceutics13040520
Xu Y, Fourniols T, Labrak Y, Préat V, Beloqui A, des Rieux A. Surface Modification of Lipid-Based Nanoparticles. ACS Nano. 2022;16(5):7168-7196. doi:10.1021/acsnano.2c02347
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.