Various types of nanomaterial have been explored for applications in vaccines as adjuvants and delivery vehicles, particularly liposomal formulations, which have shown great success in delivering mRNA instructing for the transcription of the SARS-CoV-2 (COVID-19) spike protein within several COVID-19 vaccines.

Image Credit: Unitone Vector/Shutterstock.com
Given the novelty and additional challenges involved with mounting sensitive vaccine materials onto nanoparticle carriers, a lack of standardized characterization of nanovaccines limits reporting and clinical transition, and thus some of these unique considerations will be discussed here.
How Are Nanovaccines Designed?
Generally, nanovaccines consist of four functional components: an immunogen that stimulates an immune response, such as the SARS-CoV-2 spike protein; an adjuvant, which helps to drive an immune response to the immunogen; the delivery agent, such as liposomal nanoparticles; and the administration method, such as via subdermal injection, implant, or microneedle.
Various features of each component and the complete vaccine must be characterized in depth before, during, and following manufacture to ensure safety and efficacy, mainly when the platform or some other aspect of the vaccine is novel or innovative and thus under heightened scrutiny. As relatively recent additions to the clinical arsenal, nanovaccines present several special considerations during manufacture and subsequent storage and distribution.
A vaccine's immunogenic component may originate from various processes; for example, live-attenuated vaccines culture a modified form of the virus itself, inactivated vaccines utilize the viral structure without functional components, and recombinant protein vaccines utilize a protein separately manufactured in bacteria, to name a few.
The starting material or substrate on which each specific immunogen is grown varies significantly with type. However, important properties that must be characterized and are common to all methods include yield, cost, risk of contamination, scalability, effects of post-translational modifications, complexity, and timescale, all of which determine the feasibility of large-scale vaccine manufacture and commercialization.
Subsequent purification of the immunogen can also be achieved by several methods, depending on the type. Generally, various types of chromatography can be used to separate the desired product from undesired side-products, and tandem chemical characterization methods such as mass spectrometry are used to confirm purity.
Next, the immunogen must be attached to or encapsulated within the nanomaterial delivery component of the vaccine, which for liposomal nanocarriers usually includes one of three techniques: mechanical film hydration, solvent displacement, or detergent depletion.
The thin-film method (mechanical film hydration) is perhaps the most widely employed method of liposome preparation. It involves using a rotary evaporator to form a thin film on the inner side of the glass, followed by mechanical separation by shaking and sonication to allow the lipid film to peel away from the wall and form liposomes.
Bilayer liposomes better form above a particular transitional temperature, and thus the lipid wall is heated with a water/buffer solution containing the immunogen in the rotary flask, ensuring that the lipid bilayer encapsulates the functional component of the vaccine. This method is highly reproducible but offers a relatively low encapsulation yield compared to other methods.
Importantly, many immunogens are liable to be highly temperature sensitive, particularly unprotected nucleic acid and protein strands, and thus the method of encapsulation must avoid high temperatures in these cases. Similarly, harsh solvents that may damage the cargo or present a direct human health risk if allowed to linger following manufacture must be avoided.
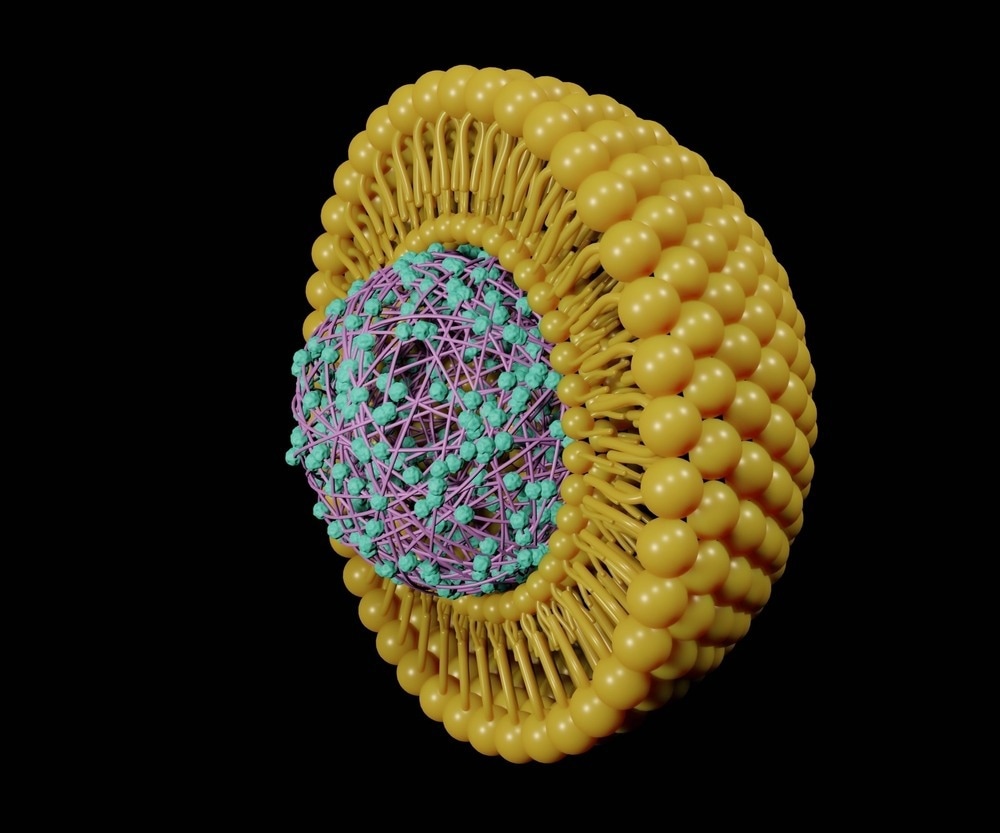
Image Credit: Love Employee/Shutterstock.com
How is the Final Nanovaccine Product Characterized?
Whichever method of encapsulation is utilized, it must be scalable to meet market demand and provide consistent immunogen loading while presenting identical physicochemical characteristics as one another. Properties such as nanoparticle size and surface chemistry play a role in biodistribution and the ability of the nanocarrier to cross cell membranes, and thus liposomal nanovaccine quality control includes size normalization steps, often utilizing pore-exclusion filters or extruders that allow liposomes of set diameter to be generated.
Nanoparticle size and homogeneity can be characterized by sizing techniques such as dynamic light scattering or nanoparticle tracking analysis, which utilizes light scattering measurements combined with knowledge of particle density and optical characteristics to establish particle diameter. Very large liposomes are visible under a light microscope, though for high-resolution images of nanovaccines, microscopes utilizing incident electrons must be used, which have a smaller wavelength than photons.
However, many low-density nanoparticle constructs, such as liposomes would appear transparent under an electron microscope, and the techniques are only applicable to nanoparticles constructed from materials with higher density, such as gold.
The surface charge of the lipid membrane can be characterized by zeta potential analyzer, wherein alternating electronic charges “push” and “pull” the particles to and from electrodes. With knowledge of particle density and solvent viscosity, the potential difference at the interface between the particle surface and surrounding medium can be calculated in volts.
Zeta potential is, therefore, highly dependent on the constituent ions present in the local media, and the pH of said medium, and thus characterization in relevant physiological conditions is important to establish the in vivo effect.
When optimizing nanovaccine efficacy and safety, the interaction of each vaccine component must be checked against every other component, as one may change the function of or inhibit another. For example, the permeability of a liposomal carrier can be characterized by the calcein release method, wherein the fluorescent compound calcein is co-loaded with the immunogen and release tracked using fluorescent microscopy.
Calcein is self-quenching at high concentrations, and thus only released fluorophore will be detected. A wide variety of alternative fluorophores with appropriate compatibility and suitability to the nanocarrier or immunogen are also available in cases where calcein is unsuitable.
The in vitro and in vivo immunogenicity of the nanovaccine is explored by tracking the host response, elevated levels of proinflammatory cytokines and immune cells indicative of vaccine efficacy. A plethora of specific biochemical assays can be exploited for this purpose, most of which are utilized when investigating the effectiveness of traditional vaccines.
Finally, before clinical use, the stability of the nanovaccine in storage is investigated at various temperatures to understand its safety and efficacy profile at various time points. Liposomes can be stored at -80⁰C and prove highly stable during long storage periods, though the immunogen component is likely to require different storage conditions if already incorporated.
Various buffers, stabilizers, and preservatives may also be included in the bulk vaccine and each must be justified and the concentration carefully monitored. Aseptic manufacture or sterilization steps are also employed to ensure that bacterial or fungal growth does not disrupt production or present downstream health concerns.
References and Further Reading
Cordeiro, A. S. et al. (2021). Nanovaccine Delivery Approaches and Advanced Delivery Systems for the Prevention of Viral Infections: From Development to Clinical Application. Pharmaceutics, 13(12). https://www.mdpi.com/1999-4923/13/12/2091
EU Science Hub. (2022). Fighting future pandemics: a new characterisation strategy for innovative nanovaccines. https://joint-research-centre.ec.europa.eu/jrc-news/new-characterisation-strategy-innovative-nanovaccines-2022-06-16_en
Sturm, L. & Ulrih, N. P. (2021). Basic Methods for Preparation of Liposomes and Studying Their Interactions with Different Compounds, with the Emphasis on Polyphenols. International Journal of Molecular Sciences, 22(12). https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8234105/
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.