AZoNano speaks with researchers from the Talapin Lab, University of Chicago, about a new method to synthesize MXenes. We discuss how this approach compares to existing solutions, such as a lack of toxic by-products, as well as what the future may hold for MXene materials.
Please could you introduce yourself and your current research activities?
Dmitri: I'm Dmitri Talapin, and I'm a chemist with an interest in the synthesis of new materials and understanding their physical and chemical properties. Ultimately, I'm focusing on the practical applications of new materials as I learned over the years, making something new is arguably the most exciting part of chemical research.
Di: My name is Di; I'm a Ph.D. student at Dmitri Talapin's lab.
Chenkun: My name is Chenkun Zhou. I'm currently a postdoc scholar in Dmitri Talapin's research group and my research interest is the chemistry of MXenes.
How did you become involved in this field?
Dmitri: Interestingly, I still feel myself a novice in the field of MXenes - it's definitely not the area that we've been working on for a very long period of time. Instead, my long-term interest was more like colloidal chemistry, and colloidal sciences. Around 2018 we became interested in the idea of making a colloidal dispersion of low-dimensional materials and nanomaterials in liquid metals.
We were desperately looking for material platforms that would be chemically compatible with liquid metals, which is when I came across Mxenes. At this time, I joined a field that already had thousands of active participants, brilliant people. But then I noticed something very interesting: this field is unusual, at least from my perspective. My lab noticed that, well, there is a huge opportunity to add chemical rigor and physical depths to the field. From there, we have had several, I think, very exciting success stories.
Di: When I was an undergrad student, I visited Professor Talapin's lab for summer research. At that time, I didn't work on MXenes, but I became friends with another lab member who was; he encouraged me to try to prepare MXene materials.
At that time, we were still using the traditional method, which is to etch a precursor particle called MAX phase. I thought, "Okay, this preparation of this material is so easy," and I became very interested in this area of research. I hope to bring new synthesis approaches to this field to make it more interesting so other people like me join.
Chenkun: My research interest during Ph.D. period focused on the development of a new class of low-dimensional organic metal halide hybrids with up to near-unity quantum yield. When I joined the group, we thought it could be a nice idea to use organic to play with the surface chemistry of MXenes.
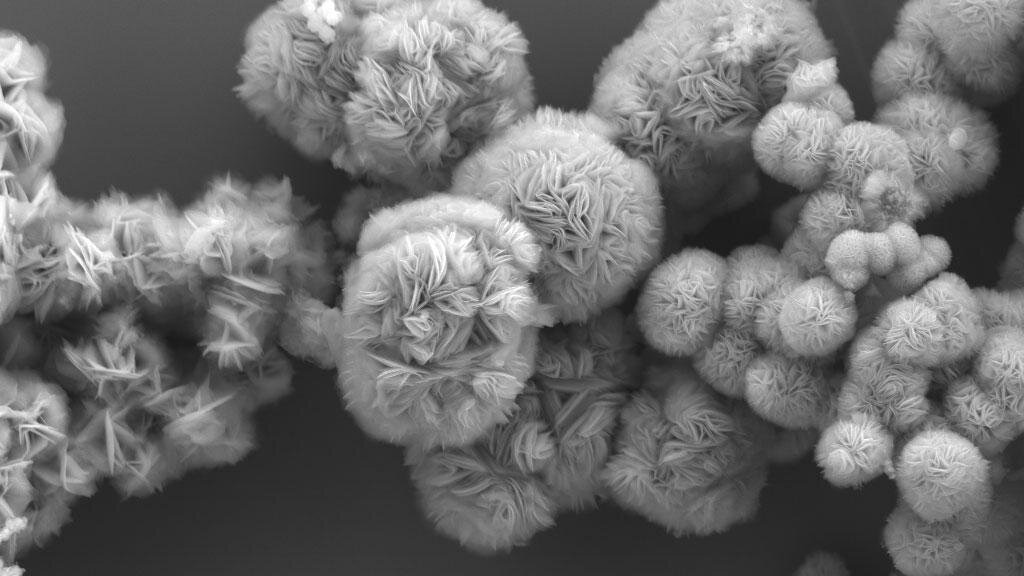
SEM Image of MXene structures. Image Credit: Di Wang/Talapin Lab
Why are MXenes such an interesting area of research? What are their highlight applications?
Dmitri: We are living in the 21st century and there are so many amazing developments, both fundamental and applied that have been already made. If you look at the field of chemistry, there are certain subareas that have been advanced to incredible depths and sophistication.
But currently, the challenging frontier is how to bridge the different subfields of chemistry, in a way that, well, solids can do things that molecules will never do. To me, MXenes look like a very unique and powerful platform for bridging organic and inorganic components, molecular and solid state components.
Di: I think the most important application of MXenes is in the field of energy storage. The discovery of MXenes came from researchers wanting to make a material for batteries or supercapacitors that enables you to intercalate ions that will carry charge to store energy electrochemically.
There are also other aspects of research showing that MXenes can make, for example, record-breaking performance in electromagnetic shielding, which has applications in defense and security as well as our home appliances.
Chenkun: For me, MXene, is the 2D material.
2D materials possess a large surface-to-volume ratio, which is quite interesting to the chemist because we can play around with its surface chemistry. Doing so allows us to improve the quality and the other properties for use in power-related applications.
What methods are typically used to synthesize MXenes?
Di: The traditional method to synthesize MXenes was developed by Professor Yury Gogotsi. Their lab developed what we call 'top-down' method, which involves firstly making a MAX phase precursor that is then broken down into MXene flakes.
This method is pretty simple in terms of handling reagents; you can just throw those precursor powders into hydrofluoric acid or HF-containing solutions and stir it for one day only at room temperature, no heating needed, to produce the MXene flakes.
However, this method produces hazardous waste and has low efficiency. To illustrate, preparing 50 pounds of MXene, which is the equivalent of what would be required to produce a Tesla car battery, would produce around 1000 liters of hydrofluoric acid-containing waste. While most labs can dispose of waste relatively easily as they are only preparing several grams of the material, scaling up to industrial levels would be problematic.
Could you discuss your recent research and its significance regarding MXene production?
Dmitri: Chemical engineering is a complex field. For example, it can be sometimes difficult to reduce a discussion to one step versus two step, as depending on the scale, the optimal method of production can be very different.
However, there is a unifying framework that chemists often think in terms of when developing new chemical methodologies: atom economy.
'Atom economy' refers to the number of atoms we need to put into our reactor and how many of these atoms will end up in the reaction product. It is a useful way to navigate and compare different synthetic processes.
From an atom economic perspective, our approach is nearly close to perfect.
Di: As mentioned, there are several issues with traditional MXene synthesis methods, particularly for industrial-scale applications. For this reason, we think that a new approach is necessary.
How did the team approach developing this new method?
Dmitri: The big picture view is that we need to have an idea and then develop it, but every project is different. Sometimes you find very old work from 1986 where they discussed a similar chemical process, that with different material systems that we could build upon.
We then realized that the chemistry developed by great John Corbett, in mid the '80s, can potentially give us a pathway towards MXenes.
From there, we've been developing it and it's become a chemical discovery process. So you do experiments, you try to understand why they fail, and then try to improve.
One key aspect of this new method is that it produces less toxic chemicals. Why is it important for the research community to explore synthesis methods that produce less toxic by-products, and what impact might this have on the overall sustainability of the field?
Dmitri: To me, MXenes is an example of nanoscience that has to be implemented on multiple levels to make a technological impact at a societal level. Of course, if you work on the scale of grams or kilograms of material synthesis, it may be less important how much toxic waste you produce.
But then again, if you want to translate these materials to applications like energy storage, the scale becomes much larger and sustainability becomes a very important part.
Regarding toxic waste, you can look at it from different perspectives: health and sustainability, or from an industry side. There are huge costs associated with the disposal of toxic waste, such as the shipping of materials containing toxic chemicals.
Regarding our method, the chemicals that are used are exactly the same chemicals that have been used for very long time, for mining titanium and for making titanium oxide particles for white pigments. Both of these processes are implemented currently on million-ton scale annually. So it means that we can take very established chemicals and very established methods, and implement them just for synthesis of MXenes in a way that has not been anticipated before.
How feasible is a future where MXenes and related materials are very heavily involved in applications like energy storage?
Dmitri: It's a difficult question to answer. I'm personally very optimistic at the moment. I believe that this research is on the rise, and in many ways MXenes will likely follow footsteps of metal organic frameworks, in a way that the more we understand about their chemistry, the more we understand about structural chemistry, functional chemistry, the more applications will emerge.
But of course, the difference between academic research and commercialization is that to successfully commercialize the material, you need to be better than any alternative along many parameters. Currently in terms of performance, in terms of manufacturability, I think we are on a very promising track.
I think there are so many areas and angles and niches that MXenes can fill, in terms of composite materials, filtration membranes, or advanced barrier materials. I'm very certain that they will be commercially used, but which exactly what will be the killer application, we will see.
Di: I'm personally optimistic. At our current stage, we are making quite an improvement in terms of making its production easier, more economical and with less waste.
Ultimately, it is difficult to predict, but we are seeing this field grow very quickly. In terms of research, more people are joining the field and I think improvements will continue to come.
Chenkun: I'm pretty optimistic, but I think there is other thing to emphasize: MXenes are not limited to energy-related applications. It takes time, and we are still in the early stage of the material, but there could be more interesting applications to be discovered in the future.
What are the next steps for your research activities?
Dmitri: My favorite direction at the moment is hybrid organic-inorganic MXenes that naturally bridge organic and inorganic materials in a completely new way, enabling us to take advantage of both the engineerability and customizability of organic molecules and electronic properties of 2D materials. Although it is in its total infancy, it probably has huge potential.
Di: As we have developed a nice method to prepare and synthesize MXenes, the next step is to make use of them and to play with them. Personally, I have been looking at using electrochemistry to process our MXene products.
Chenkun: I agree with Dmitri. The charm of these organic-inorganic hybrid materials is that you can anchor the different organics on the surface. Surface chemistry has not been done much by the community, but I think it can open up a new field for MXene chemistry.
About Prof. Dmitri Talapin
 Dmitri Talapin is Ernest DeWitt Burton Distinguished Service Professor in the Department of Chemistry, James Franck Institute, and Pritzker School of Molecular Engineering at the University of Chicago. His research interests focus on inorganic nanomaterials, from synthetic methodology to self-assembly to charge transport and optoelectronic devices.
Dmitri Talapin is Ernest DeWitt Burton Distinguished Service Professor in the Department of Chemistry, James Franck Institute, and Pritzker School of Molecular Engineering at the University of Chicago. His research interests focus on inorganic nanomaterials, from synthetic methodology to self-assembly to charge transport and optoelectronic devices.
He was born in USSR and grew up in Belarus, received a doctorate degree from the University of Hamburg, Germany in 2002, followed by a postdoctoral work at the IBM T. J. Watson Research Center. In 2005-2007, he was a staff scientist at the Molecular Foundry at Lawrence Berkeley National Laboratory and joined faculty of the University of Chicago in 2007. His recognitions include ACS Inorganic Nanoscience Award, Materials Research Society Outstanding Young Investigator Award, David and Lucile Packard Fellowship in Science and Engineering, and others. He was elected a Fellow of the Royal Society of Chemistry in 2014 and serves as an Associate Editor for Chemical Science published by the Royal Society of Chemistry.
About Chenkun Zhou, Ph.D.
 Chenkun Zhou received his B.S. degree in Chemistry from Nanjing University (2014) and Ph.D. degree in Chemical Engineering from Florida State University (2019) under the guidance of Prof. Biwu Ma. Dr. Zhou is currently a postdoc scholar in Prof. Dmitri Talapin’s research group at the University of Chicago. His research interests focus on the assembly of organic and inorganic hybrid structures, in particular organic-inorganic hybrid MXenes and low-dimensional metal halide perovskites-related materials, which unite the tailorability of organic molecules with the unique properties of inorganic components.
Chenkun Zhou received his B.S. degree in Chemistry from Nanjing University (2014) and Ph.D. degree in Chemical Engineering from Florida State University (2019) under the guidance of Prof. Biwu Ma. Dr. Zhou is currently a postdoc scholar in Prof. Dmitri Talapin’s research group at the University of Chicago. His research interests focus on the assembly of organic and inorganic hybrid structures, in particular organic-inorganic hybrid MXenes and low-dimensional metal halide perovskites-related materials, which unite the tailorability of organic molecules with the unique properties of inorganic components.
About Di Wang
 Di Wang is a PhD candidate in Dmitri Talapin lab at the University of Chicago Department of Chemistry. He pursues the synthesis and surface engineering of 2D transition metal carbides and nitrides (MXenes).
Di Wang is a PhD candidate in Dmitri Talapin lab at the University of Chicago Department of Chemistry. He pursues the synthesis and surface engineering of 2D transition metal carbides and nitrides (MXenes).
Disclaimer: The views expressed here are those of the interviewee and do not necessarily represent the views of AZoM.com Limited (T/A) AZoNetwork, the owner and operator of this website. This disclaimer forms part of the Terms and Conditions of use of this website.