Nov 8 2012
A new power-free microfluidic chip developed by researchers at the RIKEN Advanced Science Institute (ASI) enables detection of microRNA from extremely small sample volume in only 20 minutes. By drastically reducing the time and quantity of sample required for detection, the chip lays the groundwork for early-stage point-of-care diagnosis of diseases such as cancer and Alzheimer's.
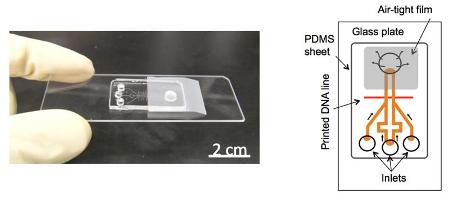 New power-free RNA detection microchip. Left: Fabricated microchip. Right: Microchannel design.
New power-free RNA detection microchip. Left: Fabricated microchip. Right: Microchannel design.
MicroRNAs (miRNAs) are small, non-coding RNA molecules that regulate gene expression in a wide range of biological processes including development, cell proliferation, differentiation and cell death (apoptosis). Concentration of certain miRNA in body fluids increases with the progression of diseases such as cancer and Alzheimer's, generating hope that these short RNA may hold the key to faster, more accurate diagnosis. Currently available techniques for sensitive miRNA detection, however, require days to reach a diagnosis and involve equipment operated only by trained personnel, making them impractical for use in many situations.
The research team set out to overcome these obstacles by developing a device that enables fast, easy-to-use point-of-care (POC) diagnosis from only a very small sample. In earlier research, the team developed a device in the form of a microchip which uses polydimethylsiloxane (PDMS), a silicone compound known for its air absorption properties, to pull reagents into a capture probe for analysis. This pumping technique simplified design by eliminating the need for external power sources, but the device required a quantity of sample too large for practical applications.
The new device also uses PDMS as an air pump, but drastically improves the method's sensitivity through a signal amplification method called laminar flow-assisted dendritic amplification (LFDA). First, DNA fragments which bond to specific miRNA sequences are fixed to a glass surface along with the miRNA sample to be analyzed, and then sandwiched under a layer of PDMS with channels in it (Figure 1). Emptied of air in a vacuum, the PDMS layer induces a pump effect which pulls amplification reagents, inserted at the channel inlets, into the channels and into contact with the miRNA, creating fluorescence-labeled dendritic structures that grow over time and can be quickly detected.
The sensitivity of this technique drastically reduces the sample quantity required for diagnosis to only 0.25 attomoles (10-18 mole), a thousand-fold improvement over the team's earlier model. Together with its detection time of only 20 minutes, these properties make the self-powered device ideal for use in resource-poor environments, promising portable point-of-care diagnosis for millions in developing countries and around the world.