Jan 25 2016
The transfer of triplet exciton energy from semiconductor nanocrystals to molecular acceptors that are surface-bound has been outlined by North Carolina State University researchers. This transfer extends the duration of the excited state that was initially developed by six orders of magnitude. The discovery is applicable in a wide range of fields such as photochemical synthesis, solar energy conversion, light therapy for treating cancer, and optoelectronics.
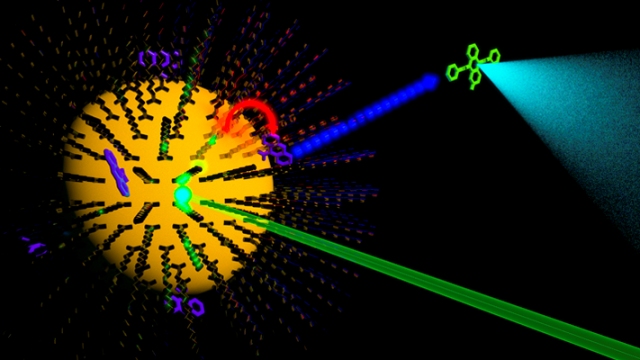 Illustration depicting semiconductor nanocrystal to molecule triplet energy transfer and established subsequent reactions.Image Credit: Cedric Mongin.
Illustration depicting semiconductor nanocrystal to molecule triplet energy transfer and established subsequent reactions.Image Credit: Cedric Mongin.
Excitons are the electron/hole pairs produced in semiconductor nanocrystals once light has been absorbed and stored temporarily as chemical energy. For example, in solar cells, the excitons send energy via the material, and this helps to collect and convert the energy into electricity.
Based on the field of photochemistry, one major disadvantage of using semiconductor nanocrystals as photosensitizers is their short excited state lifespan, typically tens of nanoseconds. The short duration means the semiconductor nanocrystals are not suitable to carry out photochemical reactions. NC State chemistry professor Felix Castellano, along with postdoc Cedric Mongin and graduate student Sofia Garakyaraghi, discussed the possibility of extending the excited state lifespan to a time duration sufficient to perform chemistry.
The fundamental question was, ‘Can we take a nanoparticle excited state with a lifetime of tens of nanoseconds and extend it through sensitization.’ If we take the original nanocrystal excited state and transfer its energy to a triplet acceptor on the surface of the nanomaterial, then the molecular triplet excited state you create should have a long enough lifetime to promote chemical reactions. This would also suggest that semiconductor nanocrystals exhibit molecular-like behavior.
Felix Castellano, Chemistry Professor, North Caroline State University
Cadmium selenide (CdSe) nanocrystals covered with oleic acid were used by Castellano’s team. The nanocrystals were prepared by Professor Mikhail Zamkov and his graduate student Natalia Razgoniaeva at Bowling Green State University. A small quantity of the oleic acid is replaced by the molecular triplet acceptor 9-anthacenecarboxylic acid (ACA). The exciton formed in the CdSe is sent to the ACA, when a green laser pulse strikes the CdSe nanocrystal containing ACA. This is followed by the formation of molecular triplet exciton with a millisecond lifespan. This is considered to be a lifetime extension of six orders of magnitude, bringing about appropriate chemical reactivity.
The other benefit is that by translating the exciton away from the nanoparticle surface, instead of involving the nanoparticle itself in the desired chemical reactions, you won’t degrade the nanoparticle. It can keep absorbing light and transferring the energy into the bulk solution.
Felix Castellano, Chemistry Professor, North Caroline State University
The findings are reported in Science. The U.S. Department of Energy (DE-AC02-06CH11357) and the Air Force Office of Scientific Research (FA9550-13-1-0106) supported this research.