Dec 16 2016
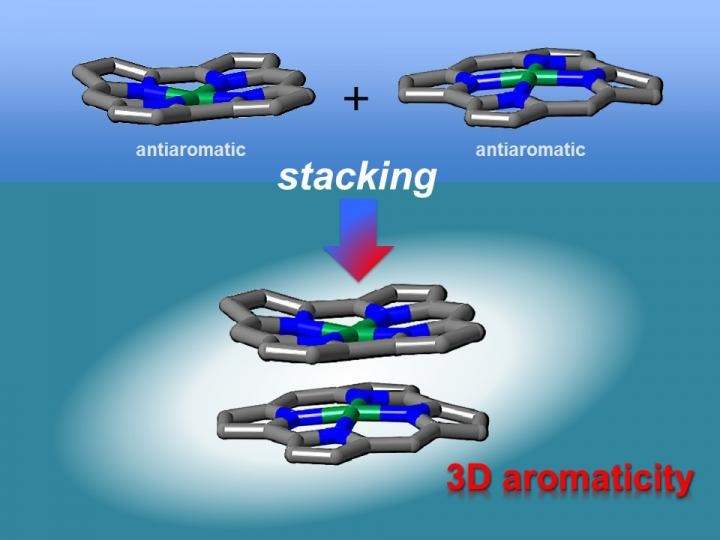 Antiaromatic planar norcorrole molecules form close face-to-face stacked structures with increased aromaticity. This behavior is quite different from that of planar aromatic molecules. This result is the first experimental proof for the theoretical prediction that the stacking of antiaromatic molecules may result in the formation of materials with three-dimensional aromaticity. (CREDIT - Nagoya University)
Antiaromatic planar norcorrole molecules form close face-to-face stacked structures with increased aromaticity. This behavior is quite different from that of planar aromatic molecules. This result is the first experimental proof for the theoretical prediction that the stacking of antiaromatic molecules may result in the formation of materials with three-dimensional aromaticity. (CREDIT - Nagoya University)
Aromatic molecules are made up of planar carbon-based rings with alternating single and double (π) bonds. These molecules possess 4n+2 (n = 0, 1, 2 ...) π electrons. π electrons are those involved in π bonds, which causes high stability because the π electrons delocalize over the ring structure.
Aromatic molecules can interact via offset π-π stacking, and the overlap of π orbitals in aromatic structures with π-π stacking can enable electron conduction, making such materials appealing for use in electronics. The overlap between π orbitals would be enhanced if π-π stacking was face-to-face rather than offset. However, face-to-face stacking is energetically unsuitable in aromatic molecules due to the repulsion of π electrons.
Theoretical researches have shown that face-to-face interactions between molecules may be accomplished using antiaromatic materials. Antiaromatic molecules possess 4n (n = 1, 2 ...) π electrons, which make them very unstable. It has been proposed that the 2D stacking of antiaromatic materials may result in the development of materials with 3D aromaticity. However, this had not been verified experimentally as antiaromatic materials are tough to synthesize due to their instability.
Recently, an international collaboration led by researchers at Nagoya University managed a breakthrough in 2D stacking of antiaromatic materials. They synthesized nickel complexes of antiaromatic planar norcorrole macrocycles. The research was reported in Nature Communications.
"We synthesized stable antiaromatic nickel norcorroles and then investigated their interactions," first author Ryo Nozawa says.
X-ray diffraction analysis revealed that the norcorrole complex stacked to create a "triple-decker" structure with the norcorrole planes were much closer together than seen in typical π-π stacking interactions. The triple-decker structure displayed aromatic attributes, in contrast to its norcorrole subunits. The researchers then fabricated a molecule possessing two antiaromatic norcorrole units connected by a flexible bridge.
Our characterization results indicate that the two norcorrole units assume face-to-face interactions to form a molecule with higher aromaticity than that of the norcorrole subunit. That is, there is strong three-dimensional electronic communication between the norcorrole subunits.
Hiroshi Shinokubo, Nagoya University
The stacking of antiaromatic units enabled closer interactions than that realized when stacking aromatic units together, confirming theoretical predictions. The resulting materials had very close π-conjugated systems, which are likely to result in large intermolecular orbital interactions. Therefore, these materials are appealing for use in optoelectronics.
The researchers also discovered that the stacked antiaromatic materials displayed nonlinear optical properties that were regulated by the creation of supramolecular structures. A material is said to possess nonlinear optical properties when it does not react linearly to the electric field of light.
Such materials are appealing for application in nanofabrication and photodynamic therapy, implying probable future applications of norcorrole-based compounds.
The article "Stacked antiaromatic porphyrins" was published in Nature Communications (DOI: 10.1038/ncomms13620). The authors of the papers include Ryo Nozawa, Hiroko Tanaka, Designated Associate Prof. Ji-Young Shin, Prof. Stephan Irle, and Prof. Hiroshi Shinokubo, Nagoya Univesity, with their international research group including Yonsei University (South Korea) and Western Washington Univesity (USA). The research received funding from the Grant-in-Aid for Scientific Research on Innovative Areas (2601): π-System Figuration, JSPS KAKENHI grant, and the Program for Leading Graduate Schools 'Integrative Graduate Education and Research in Green Natural Sciences' from MEXT, Japan, and others.