For instance, metal atoms can be made into nanoscale ‘molecular wires.’ Also known as Extended Metal Atom Chains (EMACs), molecular wires are one-dimensional chains of single metal atoms connected to an organic molecule, called a ligand, that acts as a support. Molecular wire-type compounds have a diverse array of potential uses, from LED lights to catalysts.
Researchers at the Okinawa Institute of Science and Technology Graduate University (OIST) have found a simple way to create copper molecular wires of different lengths by adding or removing copper atoms one by one. “This is the first example of a molecular copper wire being formed in a stepwise, atom-by-atom process,” says Julia Khusnutdinova, head of the OIST Coordination Chemistry and Catalysis Unit. “Our method can be compared to Lego construction in which you add one brick at a time,” she says.
Molecular wires can vary in length, with different lengths having different molecular properties and practical applications. At present, the longest EMAC reported in the literature is based on nickel and it contains 11 metal atoms in a single linear chain.
Creating molecular wires of different lengths is difficult because it requires a specific ligand to be synthesized each time. The ligand, which can be seen as an ‘insulator’ by analogy to the macro world, helps the wires to form by bringing the metal atoms together and aligning them into a linear string. However, creating ligands of different lengths can be an elaborate and complicated process.
The OIST researchers have found a new way to overcome this problem. “We have created a single dynamic ligand that can be used to synthesize multiple chain lengths,” says Dr. Orestes Rivada-Wheelaghan, first author of the paper. “This is much more efficient than making a new ligand each time,” he says.
In their paper, published in Angewandte Chemie International Edition, the researchers describe their new stepwise method of creating copper molecular wires. “The ligand opens up from one end to let a metal atom enter and, when the chain extends, the ligand undergoes a sliding movement along the chain to accommodate more metal atoms,” says Prof. Khusnutdinova. “This can be likened to a molecular accordion that can be extended and shortened,” says Rivada-Wheelaghan. By adding or removing copper atoms one at a time in this way, the researchers can construct molecular wires of different lengths, varying from 1 to 4 copper atoms.
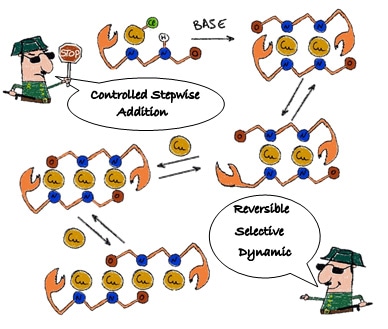
A cartoon by Dr. Rivada-Wheelaghan shows the simple stepwise process of copper atom chain synthesis using a dynamic ligand. Copper atoms can be added or removed one by one to make chains of different lengths.
This dynamic ligand offers a new way for chemists to synthesize molecules with specific shapes and properties, creating potential for many practical applications in microelectronics and beyond.
“The next step is to develop dynamic ligands that could be used to create molecular wires made from other metals, or a combination of different metals,” says Dr. Rivada-Wheelaghan. “For example, by selectively inserting copper atoms at the termini of the chain, and using a different type of metal at the center of the chain, we could create new compounds with interesting electronic properties,” says Prof. Khusnutdinova.