Spinal cord injuries can affect between 250,000 and 500,000 people annually worldwide and with an estimation of 17,000 diagnoses of spinal cord injuries per year in the United States, this disorder requires critical therapeutic research.
There are two types of major spinal cord injuries, which include transaction of the spinal cord as well as contusion of the spinal cord. These can both cause disabilities with neurological consequences.
A contused spinal cord is the result of the cord being crushed with a portion that is partially spared, such as the ventral nerve fibers. The severity of this condition is assessed through the associated physiological status of the nerve fibers that are still active; this consists of gauging whether action potentials can be passed through these fibers effectively, with the appropriate response being measured, or if these are lost due to damage of nerve fibers.
A spinal cord injury such as a contusion can cause nervous tissue damage as well as loss of function in the sensory and motor neurons. Research into repairing tissue function after a spinal cord contusion is still being studied, however, while this has been theoretically noted to be possible, there are no documented treatments within the literature.
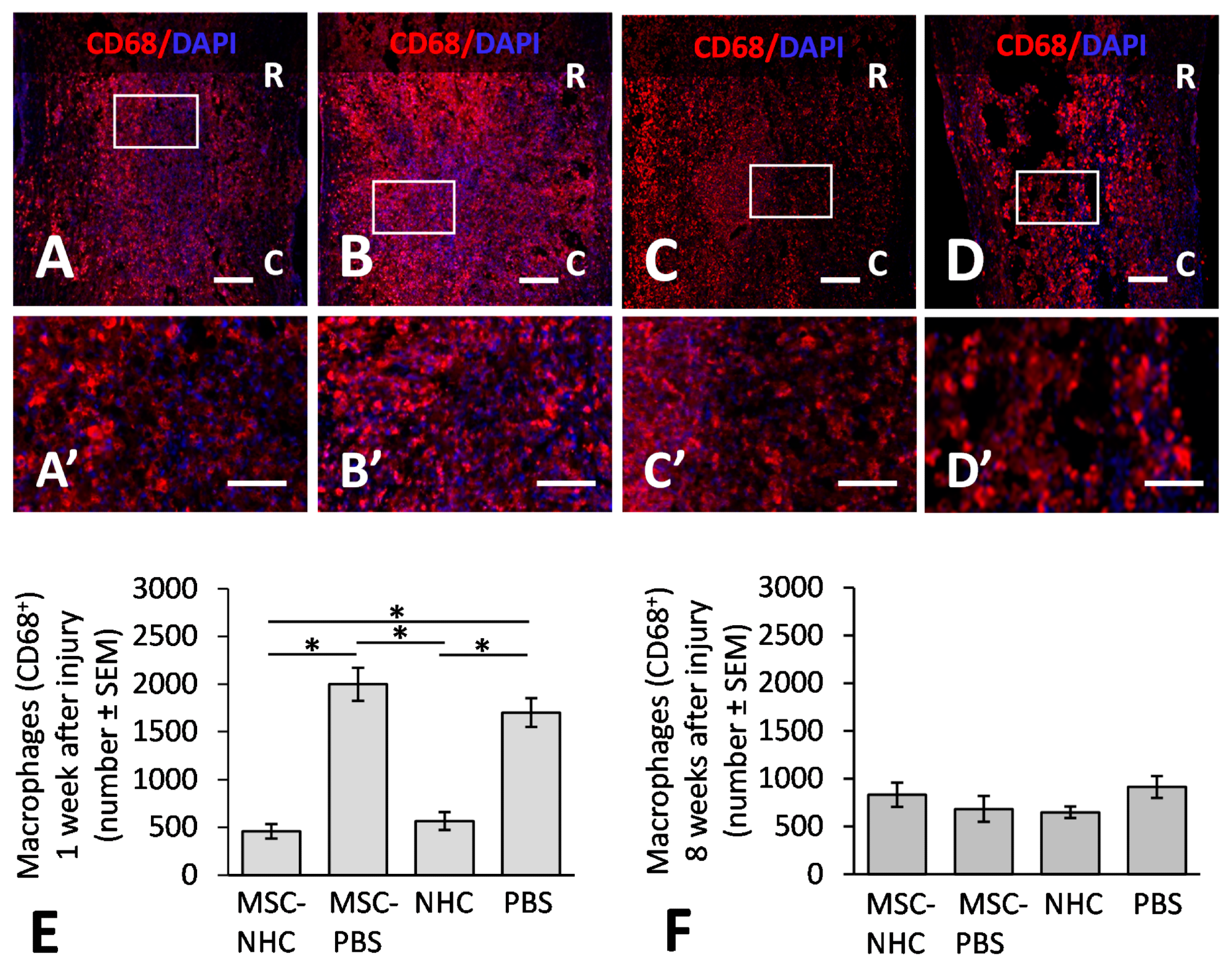
Figure 1. CD68 inflammatory response in the injury site. Photomicrographs showing macrophages stained for CD68 (red), a pan-macrophage marker, and the nucleus marker, DAPI (blue), in the injury site with MSC-NHC (A), MSC-PBS (B), NHC (C), or PBS (D) at 1 week after injury. Images in panels (A’–D’) are enlargements of the outlined area in panels (A–D), respectively. Scale bar in (A,C,D) is 250 µm, and 200 µm in (B). The scale bar in (A’–D’) is 100 µm. In (A–D), the rostral (R) and caudal (C) orientation of the horizontal sections are indicated. (E) Bar graph showing the average number of CD68+ macrophages in the injury site of each group at 1 week after injury. There are significantly less macrophages in the injury site with MSC-NHC and NHC only compared with MSC-PBS and PBS only. (F) Bar graph showing the average number of CD68+ macrophages in the injury site of each group at 8 weeks after injury. The differences in numbers of CD68+ macrophages in the injury site of the four groups were not statistically significant. In both graphs, the asterisks indicate p < 0.05, and the bars represent SEM. Abbreviations: DAPI = 4′,6-diamidino-2-phenylindole; MSC = mesenchymal stromal cells; NHC = nanofiber-hydrogel composite; PBS = phosphate-buffered saline; SEM = standard error of the mean. © Haggerty, A., et al (2022)
The field of regenerative medicine has been revolutionary for repairing tissue damage for various disorders and diseases and has become the highest priority for researchers when considering advanced treatment approaches.
Previous research has illustrated that a transplant of bone marrow-derived mesenchymal stromal cells (MSCs), within the site that was injured, can lead to repair of the nervous tissue, with possible subsequent improvement in the tissue functionality. With these cells being rich in paracrine factors that can be released to aid immunomodulation, neuroprotection and enhance the growth of axons, their potential for regeneration of damaged nervous tissue may be revolutionary.
A group of international researchers has collaborated on a study that has undertaken this innovative investigation on spinal cord injury repair, which utilizes nanotechnology as a means to advance the field of regenerative medicine.
Innovative Research
This research group utilized an injectable nanofiber-hydrogel composite (NHC), which was shown to result in nervous tissue repair in an adult rat model with a contusive spinal cord injury.
The nanofiber-hydrogel composite was constructed with poly(ε-caprolactone) (PCL) nanofibers, which were bound to a hydrogel network that was produced from thiolated hyaluronic acid (HA-SH) and poly(ethylene glycol) diacrylate (PEGDA). This composite was mixed with the MSCs that were harvested from the bone marrow of rats before being injected into the spinal cord contusion site.
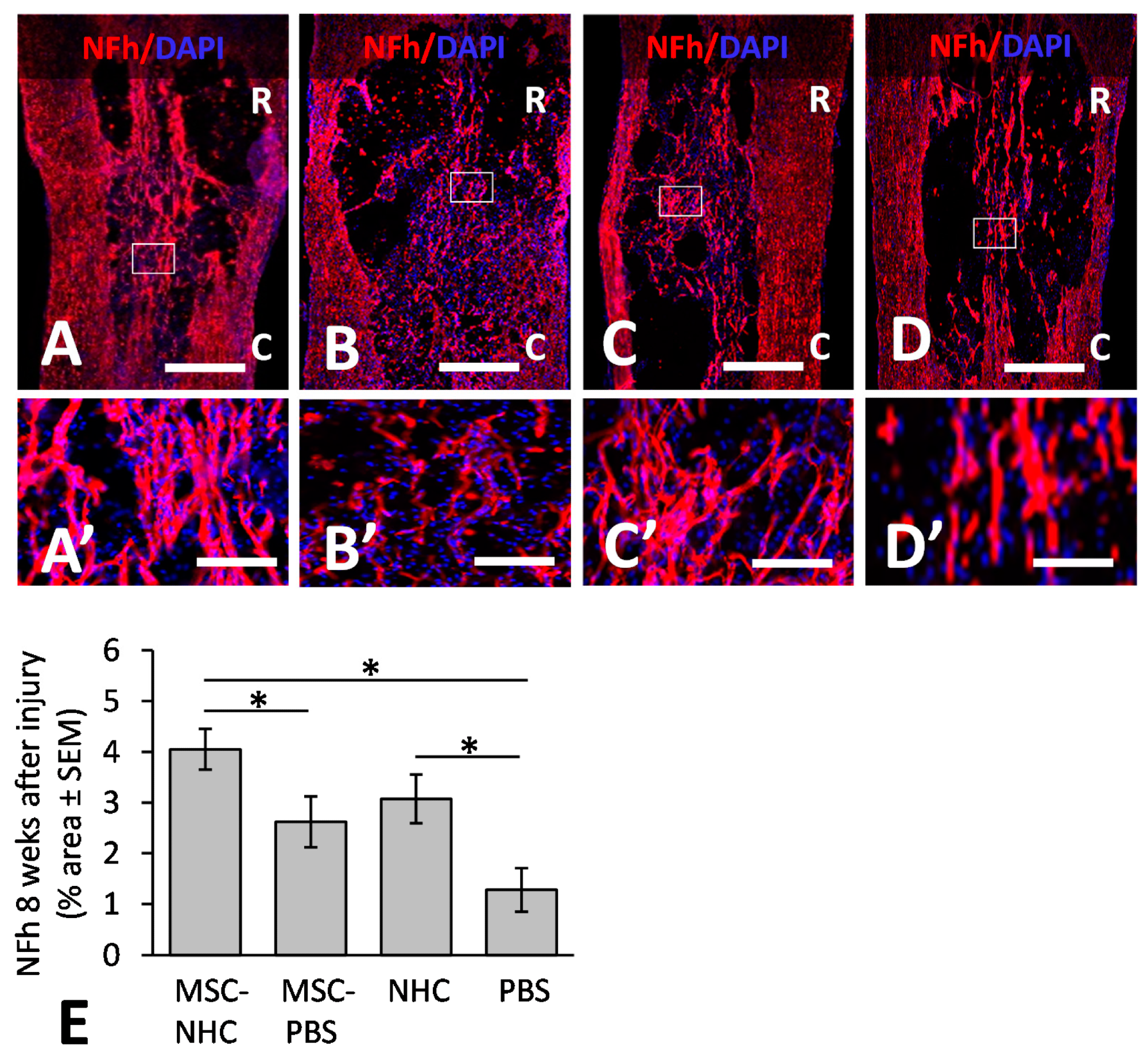
Figure 2. Axon presence in the injury site. Photomicrographs showing axons stained for NFh (red) in the injury site with MSC-NHC (A), MSC-PBS (B), NHC (C), or PBS (D) at 8 weeks after injury. Sections were counterstained with the nucleus marker, DAPI (blue). Images in panels (A’–D’) are enlargements of the outlined area in panels (A–D), respectively. The scale bar in (A–D ) is 450 µm. Scale bar in (A’,C’) is 125 µm, and 115 µm in (B’,D’). In (A–D), the rostral (R) and caudal (C) orientation of the horizontal sections are indicated. (E) Bar graph of the percentage area of injury site positive for NFh at 8 weeks after injury. There were significantly more NFh+ axons in the injury site with MSC-NHC compared with MSC-PBS and PBS only. Asterisks indicates p < 0.05. Bars in the graph represent SEM. Abbreviations: DAPI = 4′,6-diamidino-2-phenylindole; MSC = mesenchymal stromal cells; NFh = neurofilament high molecular weight; NHC = nanofiber-hydrogel composite; PBS = phosphate-buffered saline; SEM = standard error of the mean. © Haggerty, A., et al (2022)
This nanocomposite was found to have a similar storage modulus as the spinal cord, which refers to the measure of the solidity of a material, as when the storage modulus is high, there is a higher level of difficulty to break it down. This factor can be used as a measure of energy that is required to break down a material. Additionally, the composite can also provide high porosity for supporting the infiltration of the host cell, as well as other cell processes such as migration.
The findings of this study demonstrated the potential of advancing nervous tissue repair, with the nanofiber-hydrogel composite facilitating tissue formation within the spinal cord injury, as well as disarming the immune-inflammatory response, with an enhancement of anti-inflammatory and pro-regenerative responses.
The role of macrophages was found to be significant in this process, with macrophages being a necessary component during spinal cord injuries for clearing tissue debris; however, due to also secreting cytotoxic molecules, this cell can contribute to nervous tissue damage. This study was found to decrease the volume of macrophages within the site of injury through the use of the nanofiber-hydrogel composite.
Significance and Future Outlook
The loss of nervous tissue after the primary injury is a characteristic of spinal cord injuries; however, secondary damage can occur due to cytotoxic cascades, which can result in further tissue and neurological damage. The use of MSC and NHC within this innovative research can reduce this occurrence through the secretion of neutrophils, which hold a neuroprotective role.
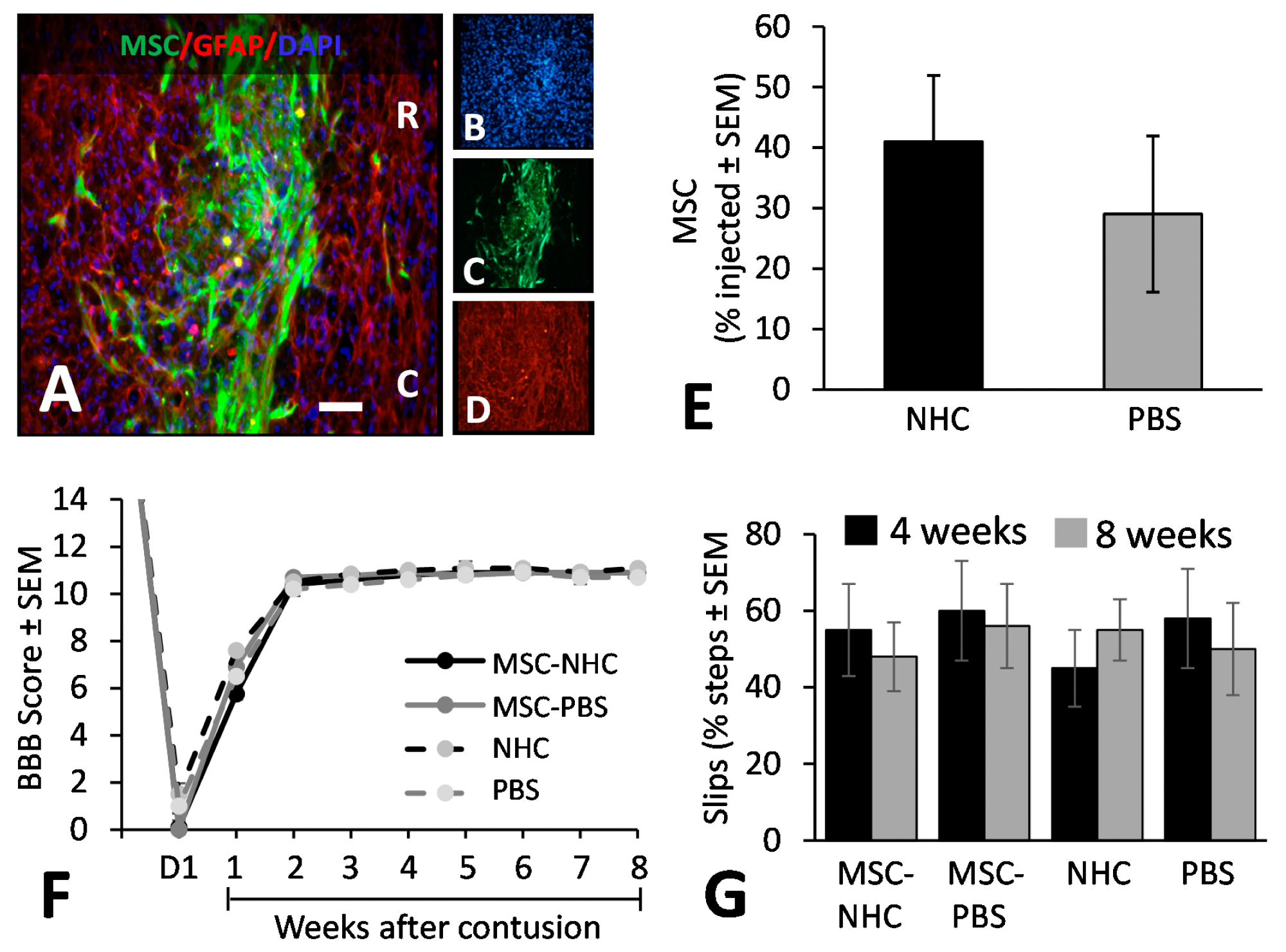
Figure 3. MSC transplant survival and hindlimb function. (A) Photomicrograph showing MSC (green), astrocytes (red, anti-GFAP), and cell nuclei (blue, DAPI) in the injury site with MSC-NHC at 1 week after injury. The scale bar in (A) is 180 µm. Scale bar in (B–D) is 550 µm. In (A), the rostral (R) and caudal (C) orientation of the horizontal section are indicated. (E) Bar graph showing the number of MSCs present in the injury site 1 week after injury. The difference in number between groups was not significant. (F) Line graph showing the BBB scores of the experimental groups on day 1 (d1) after injury and weekly thereafter. Differences between groups per time point were not statistically significant. In both graphs, the bars represent SEM. (G) Bar graph showing the total slips as a percentage of the total steps made by the rats to cross the horizontal ladder at 4 and 8 weeks after injury. Differences between groups per time point were not statistically significant. In all graphs, the bars represent SEM. Abbreviations: BBB = Basso, Beattie, Bresnahan; d = day; DAPI = 4′,6-diamidino-2-phenylindole; GFAP = glial fibrillary acidic protein; MSC = mesenchymal stromal cells; NHC = nanofiber-hydrogel composite; PBS = phosphate-buffered saline; SEM = standard error of the mean. © Haggerty, A., et al (2022)
The researchers have stated that the next stage of research would entail furthering the understanding of the mechanisms of MSC and NHC, which can be used to explore applications with other cell types. While this research is in its early phase, its implications hold great potential for both the field of regeneration medicine as well as nanotechnology, and with further research, could possibly be used in a clinical setting for advanced treatment of spinal cord injuries to aid with nervous tissue repair and prevent secondary damage.
With approximately 282,000 individuals living with this disorder in the United States, the need for advanced care for patients who have experienced spinal cord injuries is paramount to enable quality treatment as well as increase the quality of life of these patients.
Reference
Haggerty, A., Maldonado-Lasunción, I., Nitobe, Y., Yamane, K., Marlow, M., You, H., Zhang, C., Cho, B., Li, X., Reddy, S., Mao, H. and Oudega, M., (2022) The Effects of the Combination of Mesenchymal Stromal Cells and Nanofiber-Hydrogel Composite on Repair of the Contused Spinal Cord. Cells, 11(7), p.1137. Available at; https://www.mdpi.com/2073-4409/11/7/1137/htm
Further Reading
Ju, G., Wang, J., Wang, Y. and Zhao, X., (2014) Spinal cord contusion. Neural Regeneration Research, 9(8), p.789. Available at: 10.4103/1673-5374.131591
Bennett J, M Das J, Emmady PD. Spinal Cord Injuries. [Updated 2021 Aug 26]. In: StatPearls [Internet]. Treasure Island (FL). Available from: https://www.ncbi.nlm.nih.gov/books/NBK560721/
Kimbell, G. and Azad, M., (2021) 3D printing: Bioinspired materials for drug delivery. Bioinspired and Biomimetic Materials for Drug Delivery, pp.295-318. Available at: https://doi.org/10.1016/B978-0-12-821352-0.00011-3
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.