Direct production of H2O2 by mixing oxygen (O2) and hydrogen (H2) is unsafe, owing to the risks of explosion. Thus, new methods must be developed to produce H2O2 in a sustainable, cost-effective, and safe manner.
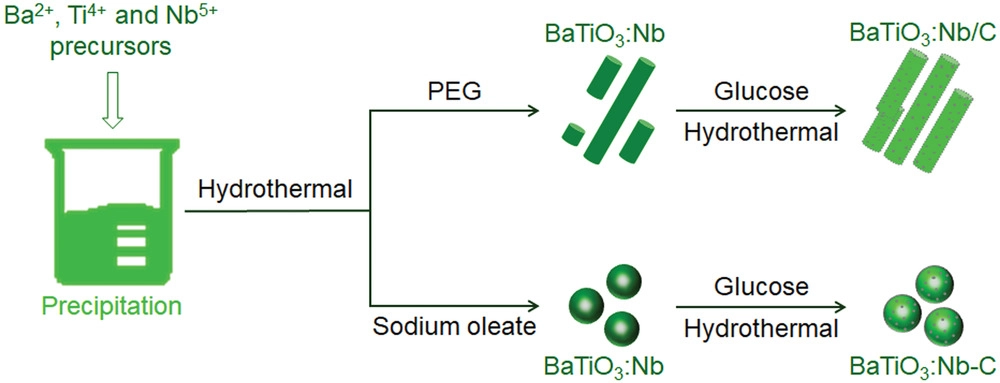
Schematic preparation processes for BaTiO3:Nb with different morphologies and modified with carbon quantum dots. Image Credit: Hedin, N et al., Advanced Science
The conversion of O2 and water (H2O) into H2O2 through the semiconductor-based photocatalysis method represents a feasible way to industrially produce H2O2. Although several semiconductor photocatalysts were developed to produce H2O2, such catalysts often produced very small amounts of H2O2 owing to the slow reaction kinetics, which necessitated the identification of new methods for photocatalytically induced and efficient synthesis of H2O2.
Recently, piezoelectrics gained considerable prominence as catalysts in redox reactions for H2O2 production and water splitting. Piezocatalysts are typically referred to the noncentrosymmetric crystals, such as bismuth ferrite and barium titanate (BaTiO3). However, the reaction rates of piezocatalysts are low due to the periodic application of mechanical force and failure of related piezopotentials to initiate the critical Gibbs free energy threshold required for redox reactions.
New Method Proposed for H2O2 Generation
The coupling of photoelectric and piezoelectric effects in piezoelectric semiconductors is a proven and powerful approach for improving the catalytic activities for a range of solar-driven reactions. The piezopotential generated when the piezoelectric semiconductor is subjected to light irradiation and mechanical energy, such as ultrasound activation, can modulate the separation and migration of photoinduced charge carriers, which considerably enhances the catalytic abilities compared to photocatalysis without piezoelectric polarization, leading to higher H2O2 production rate.
However, the energy-band structure of piezoelectric semiconductors partially determines the piezoelectric photocatalytic efficiency.
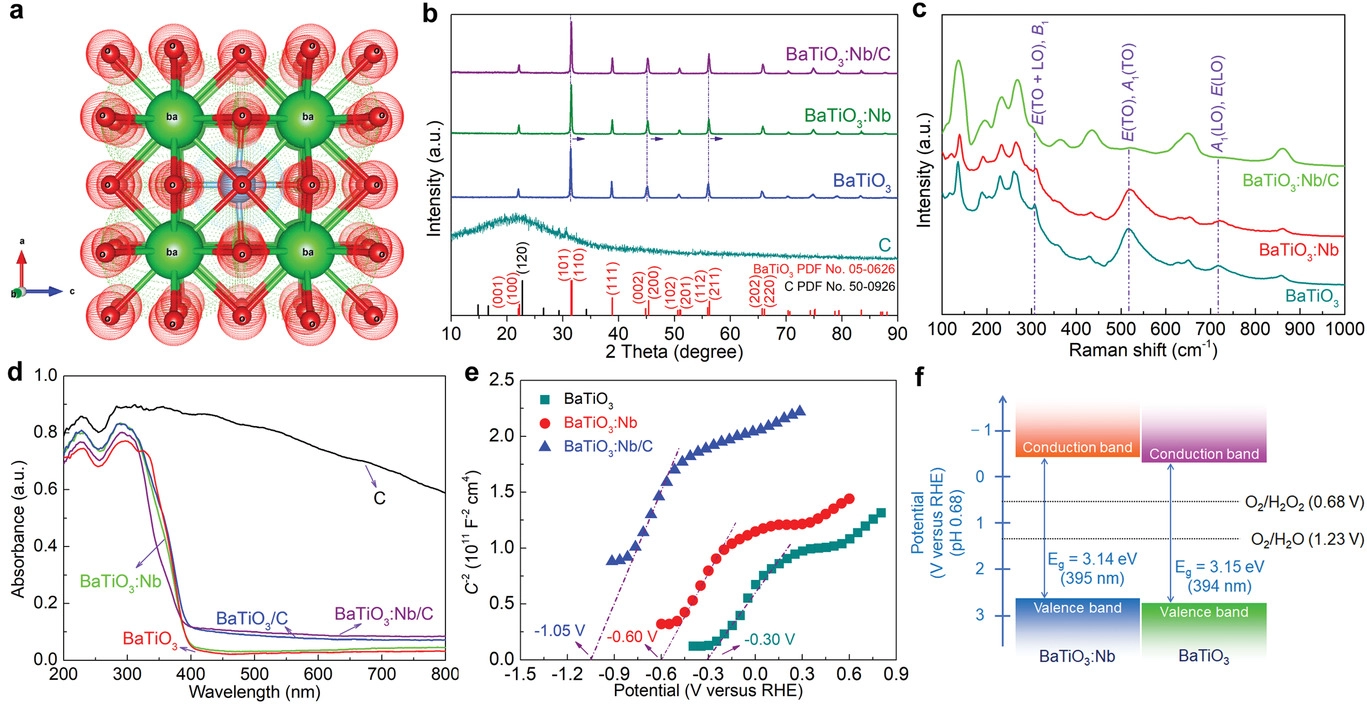
Structural and electronic properties of various catalysts. a) Crystal structure of tetragonal BaTiO3. b) X-ray diffraction patterns of pure carbon, BaTiO3, BaTiO3:Nb, and BaTiO3:Nb/C. c) Raman spectra of BaTiO3, BaTiO3:Nb, and BaTiO3:Nb/C. d) Diffuse reflectance ultraviolet–visible absorption spectra of the catalysts. e) Electrochemical Mott–Schottky curves of BaTiO3, BaTiO3:Nb, and BaTiO3:Nb/C. f) Band structures of BaTiO3 and BaTiO3:Nb. Image Credit: Hedin, N et al., Advanced Science
BaTiO3 has gained attention as a piezocatalyst owing to its exceptional piezoelectric properties. However, the wide bandgap of the semiconductor makes it unsuitable as a photocatalyst. Although doping bulk BaTiO3 with niobium (Nb) and manganese (Mn) cations can reduce the bandgap width, the method also reduces the piezoelectric polarization intensity to a considerable extent.
Moderate dopant concentrations are typically effective in enhancing both piezophotocatalytic and piezocatalytic activities. However, few studies have focused on catalytic production of H2O2 under the piezophototronic effect using Nb-doped piezoelectrics.
Carbon-based nanoparticles, such as carbon quantum dots (CQDs) that are 2-10 nanometers in size, possess robust optical absorption properties in the near-visible region. CQDs are used almost exclusively for organic dye photodegradation and as photosensitizers. Although the role of CQDs in photocatalysis remained underexplored, the nanoparticles demonstrated their effectiveness in the photoreduction of carbon dioxide to formic acid.
Synthesis, Characterization, and Evaluation of CQD-loaded Nb-doped BaTiO3
In this study, researchers synthesized Nb-doped BaTiO3 (BaTiO3:Nb) with CQD photosensitizers and evaluated its effectiveness for the piezophotocatalytic production of H2O2 under ultrasound and visible light co-irradiation.
The hydrothermal method, followed by the post-annealing method, was used to fabricate both Nb-doped BaTiO3 and undoped BaTiO3 samples. The only difference during the synthesis of undoped BaTiO3 samples was the removal of niobium pentachloride (NbCl5). Another type of Nb-doped BaTiO3 was also prepared by the same process using sodium oleate in place of poly (ethylene glycol) (PEG).
CQDs were loaded to the as-synthesized BaTiO3:Nb samples through the in-situ deposition method. BaTiO3:Nb catalysts synthesized using PEG as additive were designated as BaTiO3:Nb/C after CQD loading, while the catalysts synthesized using sodium oleate as additive were designated as BaTiO3:Nb-C.
Field-emission scanning electron microscopy and transmission electron microscopy (FE-SEM and FE-TEM), X-ray diffraction (XRD), piezoresponse force microscopy (PFM), dynamic light scattering (DLS), electron paramagnetic resonance (EPR) spectroscopy, and Raman spectroscopy were used to characterize the synthesized samples. Finite method calculations were performed using COMSOL Multiphysics 5.4 with a steady-state study-based model of a piezoelectric device.
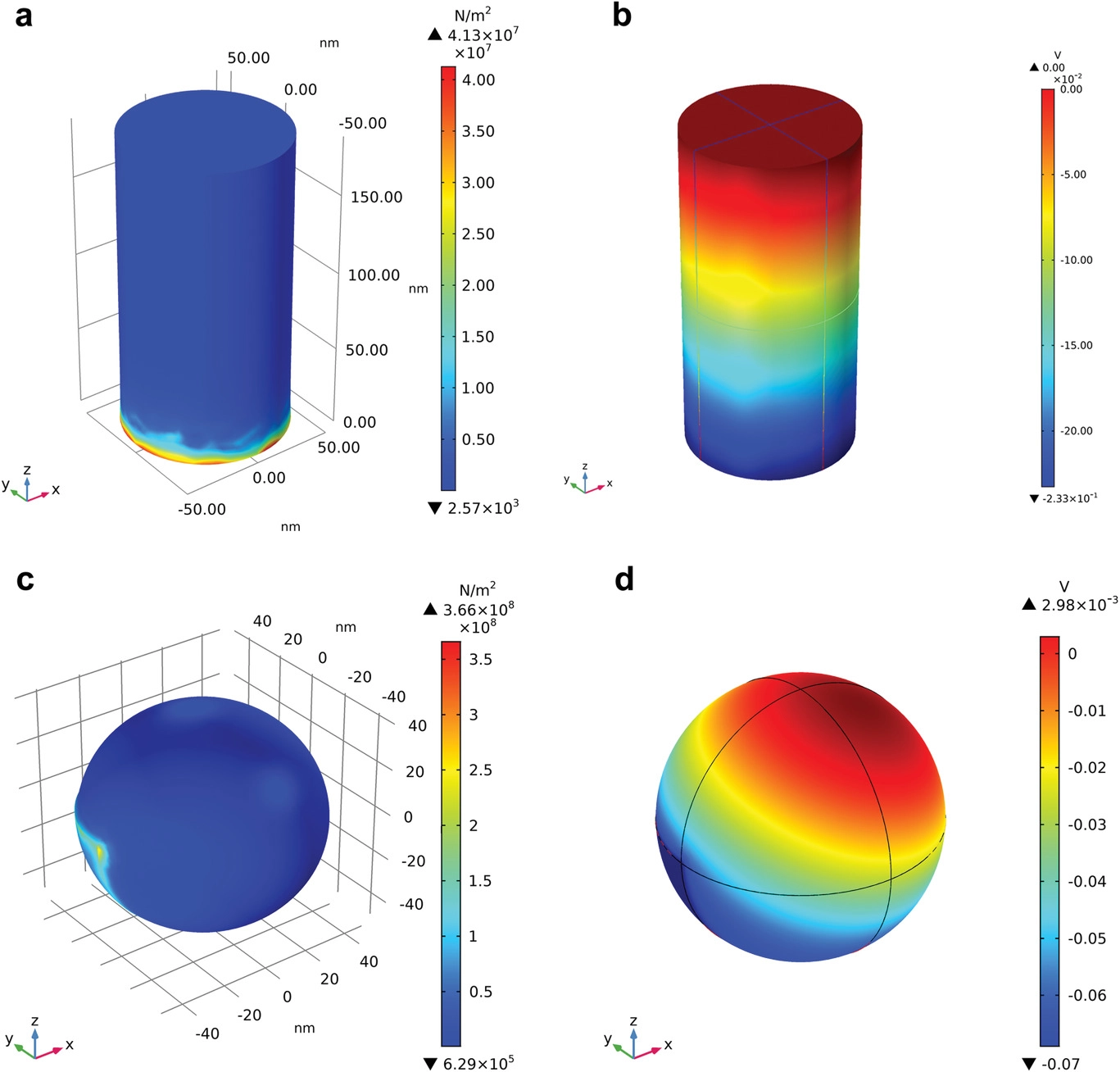
Finite element method simulation for the strain and piezopotential distribution on the surface of BaTiO3 a,b) nanorod and c,d) nanoparticle with the cavitation pressure of 108 Pa. Image Credit: Hedin, N et al., Advanced Science
Catalytic Production of H2O2
The synthesized BaTiO3:Nb/C and BaTiO3:Nb-C catalysts were mixed with a water-ethanol solution in a glass bottle and stirred in the dark for 10 minutes. The catalytic reactions were then performed under ultrasound activation with 40-kilohertz frequency, visible light irradiation with a wavelength of more than 420 nanometers, and both ultrasound and visible light co-irradiation to study piezo-, photo-, and piezophoto-catalysis reactions for the generation of H2O2, respectively. Subsequently, researchers evaluated the photocatalytic reactions for overall water splitting.
Significance of the Study
BaTiO3:Nb sensitized by CQDs were synthesized successfully. The catalyst was effective for the production of H2O2 from H2O and O2 in an ethanol solvent under visible light and ultrasound co-irradiation. The CQDs successfully absorbed visible light at wavelengths greater than 420 nanometers.
The H2O2 generation rates attained using BaTiO3:Nb/C catalysts during piezophotocatalysis were 1360 micromole per gram per hour, with 0.14 percent of solar-to-chemical conversion (SCC) efficiency in 120 minutes of piezophotoreaction. The production rate was significantly higher than sole photo- and piezo-catalysis due to the synergistic coupling of photocatalysis and piezoelectric polarization.
The H2O2 generation rates were higher in piezocatalysis compared to photocatalysis as the piezoelectric polarization field was mediated by BaTiO3:Nb, where the charge carriers from CQDs were injected into the BaTiO3:Nb conduction band. These carriers can migrate to the catalyst particle surface without recombination and induce redox reactions at the liquid-solid interface. The attribution was verified when BaTiO3:Nb/C with highly anisotropic geometries demonstrated substantially higher piezophotocatalytic activity compared to the BaTiO3:Nb-C with spherical structures.
Equation-driven calculations demonstrated that the anisotropic geometry was primarily responsible for the high piezopotential. The CQD deposition improved the polarized charge transfer and conductivity of the synthesized catalysts, which facilitated the polarized charge-induced migration of surface-screening charges and thereby improved the piezocatalytic activities.
Taken together, the findings of this study demonstrated a new and effective approach based on nanopiezoelectrics for large-scale production of H2O2 using mechanical energy and visible light that holds the potential to replace the environmentally toxic synthesis method of anthraquinone oxidation.
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.