A recent study published in the journal Nature Communications focuses on how protons move in the confined water films between MXene layers, which enables charge transportation. The findings from this study could help to improve the performance of MXenes as energy storage materials.
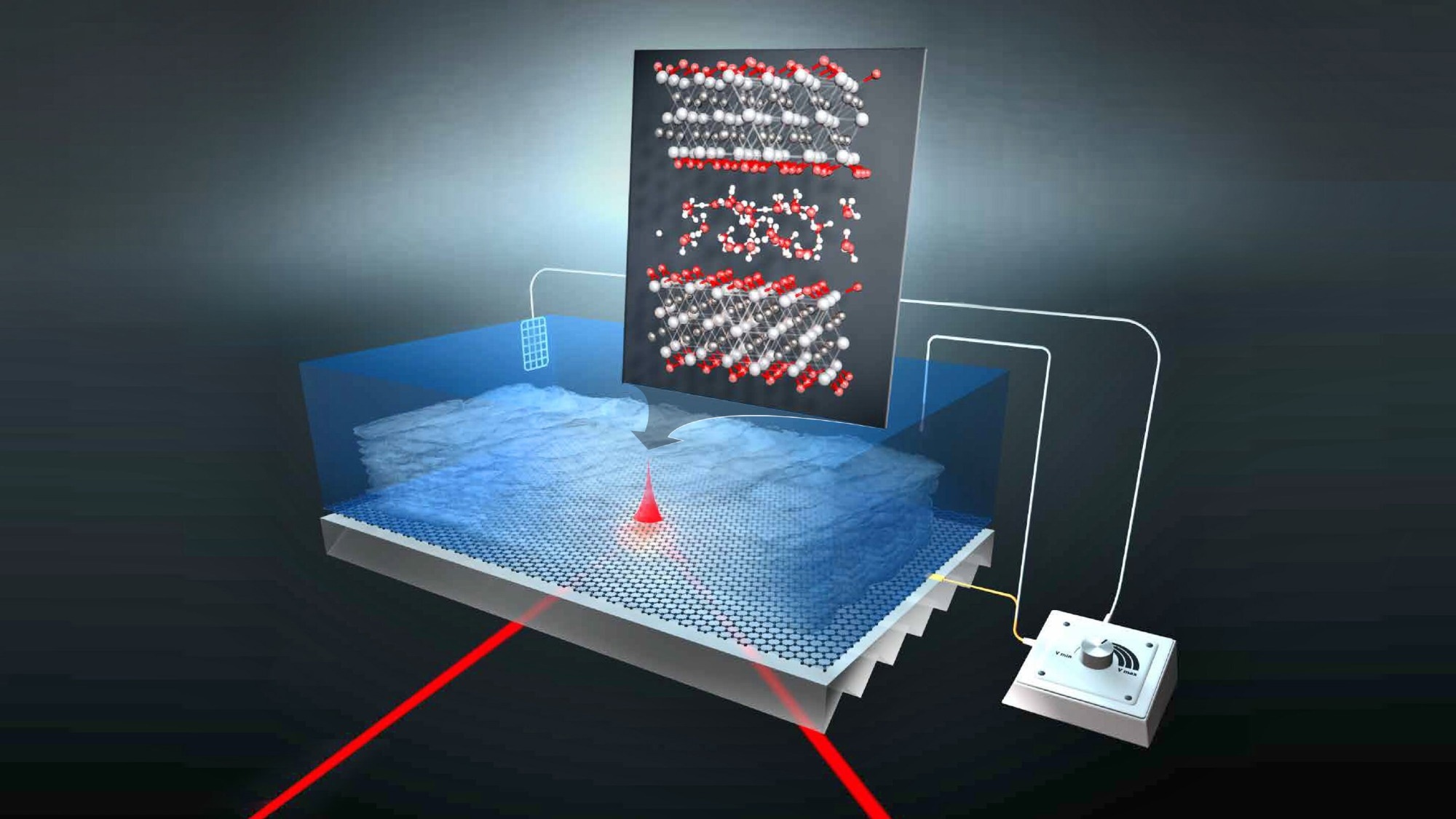
The experiment: Infrared light excites protons in the water film, which move between the Ti3C2-MXene layers. Their oscillation patterns show that they behave differently than in a thicker film of water. Credit: M. Künsting /HZB
MXenes can store electrical energy akin to batteries while also charging and discharging rapidly like supercapacitors. Their unique combination of properties makes them a promising candidate for energy storage.
Pseudocapacitors: An Innovative Solution for Efficient Energy Storage
The intermittency of renewable energy sources means that effective energy storage solutions are vital to guarantee the stability and reliability of the energy grid. However, the existing energy storage technologies, such as conventional batteries and supercapacitors, have limitations that restrict their ability to provide a comprehensive solution.
While batteries can store large amounts of energy, their slow charging and discharging processes hamper their efficiency. Supercapacitors, on the other hand, can charge and discharge rapidly, but their limited energy storage capacity restricts their use in short-term energy storage applications.
Pseudocapacitors are a new class of materials that combine the advantages of both batteries and supercapacitors. They are capable of storing large amounts of electrical energy like batteries while also being able to charge and discharge rapidly like supercapacitors.
Researchers worldwide are investigating various types of pseudocapacitive materials, including MXenes, conductive polymers, and metal oxides, to enhance their performance and efficiency further. With their remarkable energy storage capabilities, pseudocapacitors could play a vital role in transitioning towards a sustainable, low-carbon energy system.
MXenes as Pseudocapacitive Materials
Among these pseudocapacitive materials, MXenes have emerged as a promising option due to their unique properties. MXenes are a large family of conductive two-dimensional (2D) transitional metal carbides, nitrides, and carbonitrides with hydrophilic surfaces.
The most studied MXene, titanium carbide (Ti3C2Tx), has shown remarkable electrochemical properties in acidic electrolytes, making it a strong contender for pseudocapacitive energy storage materials.
However, despite significant research efforts, the mechanism behind proton intercalation in MXenes is not yet fully understood. While water confined in Ti3C2Tx MXene has been characterized by nuclear magnetic resonance (NMR) and inelastic neutron scattering, a reliable method for directly characterizing the hydrated protons remains elusive.
These challenges pose a significant barrier to the development of MXenes as pseudocapacitive energy storage materials, and their resolution is critical to unlocking their full potential.
Probing Proton Intercalation in MXenes Using Operando FTIR Spectroscopy
The study aimed to investigate the potential-induced proton intercalation and solvation phenomena in Ti3C2Tx MXenes by utilizing operando Fourier Transform Infrared (FTIR) spectroscopy.
The research team combined FTIR spectroscopy with theoretical modeling to probe the hydrated protons and water molecules confined within the interlayer arrangement of Ti3C2Tx MXenes.
This methodology allowed them to identify the fundamental differences in the H-bonding structure of hydrated protons electrochemically intercalated into 2D slits between Ti3C2Tx MXene layers compared to the bulk solution.
The use of FTIR spectroscopy to probe intercalation and solvation phenomena in MXenes is a novel approach, and the results provide a new perspective on the proton intercalation mechanism in MXenes.
"The fact that water molecules absorb infrared radiation particularly strongly while MXene emits very low amount of light in this energy range made IR spectroscopy ideally suited to our question," explains Tristan Petit, a co-author of the study.
Key Developments and Prospects of the Study
This research revealed a unique hydration structure in MXene materials, where protons are solvated by fewer water molecules under confinement compared to bulk water.
These finding shed light on the pseudocapacitive energy storage mechanisms of MXenes in acidic environments and explains the fast diffusion of protons in these materials, which has implications for their charging and discharging times.
Moreover, this work highlights MXenes as a promising platform for exploring the properties of confined chemical species, which may lead to the discovery of new chemical properties.
The researchers also introduce a valuable tool for characterizing chemical species under 2D confinement in this study, which could have far-reaching applications.
This tool can be used to probe the intercalation of different ions, molecules, and electrolytes within MXene interlayer spaces, offering new opportunities for understanding the properties of confined chemical species. These insights could be useful for designing novel materials for energy storage and other applications.
Reference
Lounasvuori, M. et al. (2023). Vibrational signature of hydrated protons confined in MXene interlayers. Nature Communications. Available at: https://www.nature.com/articles/s41467-023-36842-0
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.