Reviewed by Lexie CornerMay 12 2025
Carbyne is an extremely strong, one-dimensional carbon material that could play an important role in future electronic technologies. But because it's highly unstable and breaks apart easily, making enough of it for research has been a major challenge. Now, an international team of scientists, including researchers from Penn State, may have found a way to overcome this problem.
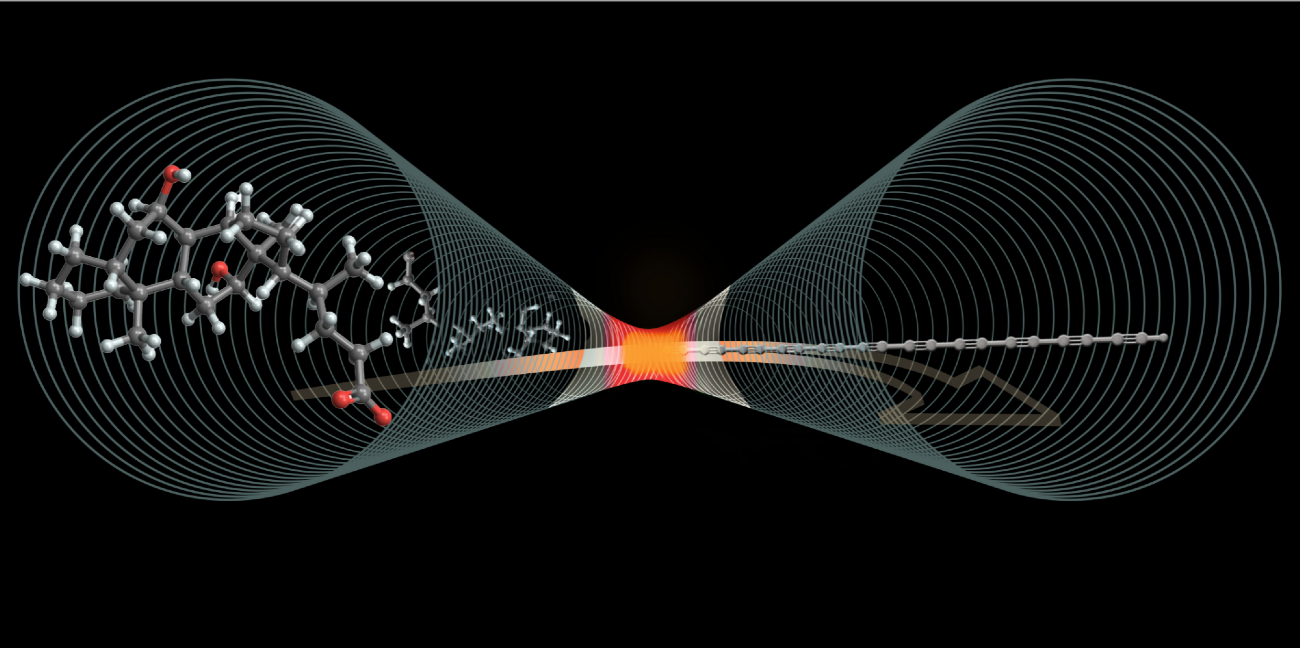 The image shows how special molecules break down when heated inside a tiny carbon tube, forming a perfectly straight chain of carbon atoms known as carbyne. Image Credit: Elizabeth Floresgomez Murray/Jennifer M. McCann
The image shows how special molecules break down when heated inside a tiny carbon tube, forming a perfectly straight chain of carbon atoms known as carbyne. Image Credit: Elizabeth Floresgomez Murray/Jennifer M. McCann
The research team has encapsulated carbyne inside single-walled carbon nanotubes - tiny, tube-shaped structures made entirely of carbon and much thinner than a human hair. They found that doing this at low temperatures improves carbyne's stability and makes it easier to produce.
This could lead to further progress in materials science and technology. The team sees it as a positive step, especially given the long-standing challenge of creating a stable form of carbyne in usable quantities for detailed research.
The history of carbyne’s discovery is like a detective story. It was predicted theoretically, but for many years, attempts to synthesize it were unsuccessful because the chains would either bend or form unintended bonds.
Slava V. Rotkin, Professor, Engineering Science and Mechanics and Study Co-Author, The Pennsylvania State University
Carbyne's natural instability has made it difficult to study and even harder to consider for practical use. Still, like graphene - a single-atom-thick carbon material already used in some electronic devices - carbyne has attracted interest because of its strength and electronic properties. According to Rotkin, researchers are especially interested in carbyne because it offers a key advantage over graphene.
“Like graphene, carbyne can allow electrons to move very quickly. However, carbyne also has something called a 'semiconductor gap,' which makes it useful for building transistors, the tiny switches that power electronics. Graphene, on the other hand, doesn’t have this gap, so it can’t be used in the same way,” said Rotkin.
A semiconductor gap is a small energy range that allows a material to act as a switch for electrical current. Pure graphene lacks this gap, so electrons can always pass through it, which limits its use in transistor design.
Although graphene can be modified to create a gap, carbyne naturally has one. This suggests that electronics built with carbyne could eventually operate faster and more efficiently than those based on silicon, and possibly with fewer technical adjustments.
Carbyne was predicted theoretically, but for many years, attempts to synthesize it were unsuccessful because the chains would either bend or form unintended bonds.
Slava V. Rotkin, Professor, Engineering Science and Mechanics and Study Co-Author, The Pennsylvania State University
The researchers' new method of making carbyne may help solve another major problem: producing it in usable amounts. Until now, only very small quantities of carbyne could be made, often under extreme conditions such as high temperatures, intense pressure, or reactive chemical environments. These challenges have limited scientists' ability to fully study the material.
The team highlights that their approach is both simpler and more effective than earlier methods. First, they used ammonium cholate, a mild starting material, to grow carbyne at much lower temperatures.
Second, they enclosed the carbyne inside single-walled carbon nanotubes, which provided better protection than the thicker, multi-layered nanotubes used in the past. Finally, their method produces much more carbyne than before, giving researchers the chance to study it more closely and assess its practical use.
“Two major advancements of this technique are its low cost and high yield. This opens the door for broader studies, both in fundamental science and moving toward real applications,” said Rotkin.
The nanotubes not only protect the carbyne from breaking down, but also keep its structure nearly intact, allowing scientists to study its properties more accurately.
Although practical uses are still being explored, Rotkin says carbyne shows great promise. Because it is a strongly correlated material, its behavior goes beyond what can be explained by classical physics. This could lead to new uses in advanced computing and nanotechnology.
“Materials like this have complex behaviors, both when they're in their normal state and when they're excited. This means we're dealing with quantum materials, which could lead to entirely new technologies,” said Rotkin.
The researchers also made an unexpected discovery. They found that cholate - a common compound derived from bile acid and used to dissolve organic substances - can be turned into carbyne chains without needing extra processing steps.
“It was a complete surprise that a common solvent like cholate can transform into the carbyne chain without any further issue. It showed how even familiar materials can take on new roles in advanced chemistry,” said Rotkin.
While there is still much to learn about carbyne, Rotkin believes this work marks an important step forward. With a stable and scalable production method, researchers can now study carbyne more thoroughly and explore its potential uses.
“In the past, the amount of material available for study was barely enough for one or two groups to confirm its existence. Now, we have the opportunity to truly understand its properties and applications,” said Rotkin.
This research was also contributed to by Bo-Wen Zhang, Xi-Yang Qiu, Qingmei Hu, Ikuma Kohata, Shohei Chiashi, Keigo Otsuka, and Shigeo Maruyama of the University of Tokyo; Yicheng Ma, Yongjia Zheng, and Rong Xiang of Zhejiang University; Aina Fitó-Parera, Dmitry I. Levshov, Sofie Cambré, and Wim Wenseleers of the University of Antwerp; Ya Feng of Dalian University; Yutaka Matsuo of Nagoya University; and YuHuang Wang and Chiyu Zhang of the University of Maryland, in addition to Rotkin. Maruyama spearheaded the research team and served as the corresponding author for the study.
The US Department of Energy and the Japan Society for the Promotion of Science funded this study.
Journal Reference:
Zhang, B., et al. (2025) Low-Temperature Synthesis of Weakly Confined Carbyne Inside Single-Walled Carbon Nanotubes. ACS Nano. doi/10.1021/acsnano.4c17104?goto=supporting-info