Jul 4 2008
RIKEN scientists, along with an international team of co-workers based in the US, UK and France, have spotted a hydrogen atom simultaneously bonding to four metal atoms, a highly unusual arrangement in this particular class of chemical compounds. The research may help to understand the structure of materials that could store hydrogen more efficiently.
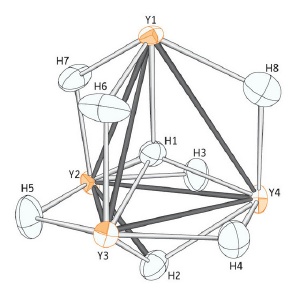 The hydrogen atom (H1) nestles perfectly between four yttrium atoms (orange) in the unusual organometallic compound.
The hydrogen atom (H1) nestles perfectly between four yttrium atoms (orange) in the unusual organometallic compound.
Hydrogen normally prefers to bond with just one other atom. However, it can be persuaded into polygamy by surrounding it with metal atoms that form a cage-like cluster.
Certain compounds of uranium or thorium are already known to host four-coordinate hydrogen atoms. But these compounds’ structures and properties are usually difficult to control or modify.
Conversely, the behavior of organometallic compounds—which possess organic (carbon-based) molecules as supporting groups around the metal atoms—can be easily controlled by changing the organic groups, allowing the compound’s properties to be fine-tuned.
Zhaomin Hou and colleagues from RIKEN’s Advanced Science Institute in Wako had previously prepared a series of organometallic compounds containing four metal atoms with eight hydrogen atoms and studied their structures by X-ray diffraction experiments1.
Now, in collaboration with the international team of co-workers, Hou and colleagues have studied a compound based on the metal yttrium using neutron diffraction2, a more precise technique which fires a stream of the neutral particles through a crystal of the compound and uses their subsequent trajectory to calculate the precise arrangement of atoms. Two crystals, both smaller than the head of a match, were analyzed at facilities in the UK and France.
The team found that hydrogen atoms in different parts of the same compound display a range of three different bonding modes—hooking up with either two, three or four other atoms. The distance between the four-coordinate hydrogen atom and its yttrium neighbors is also surprisingly small: strong evidence that hydrogen fits tightly into the cage made by the four metal atoms.
Hou says that “studies like this will increase our understanding and appreciation of the element hydrogen and its chemistry.” But it may also have practical applications. “The increasing interest in hydrogen as a fuel warrants careful studies into the structural chemistry of this most ubiquitous element,” he says, adding that there are very few high-precision studies on compounds containing multiply bonded hydrogen.
The team is now studying similar compounds containing two or more different types of metals, which are expected to have unique structures and properties of their own.
Reference
- Hou, Z., Nishiura, M. & Shima, T. Synthesis and reactions of polynuclear polyhydrido rare earth metal complexes containing “(C5Me4SiMe3)LnH2” units: A new frontier in rare earth metal hydride chemistry. European Journal of Inorganic Chemistry 18, 2535–2545 (2007).
- Yousufuddin, M., Gutmann, M.J., Baldamus, J., Tardif, O., Hou, Z., Mason, S.A., McIntyre, G.J. & Bau, R. Neutron diffraction studies on a 4-coordinate hydrogen atom in an yttrium cluster. Journal of the American Chemical Society 130, 3888–3891 (2008).