Sep 12 2008
An unusual molecule once thought to be too strained to exist has been transformed into another contorted compound by RIKEN chemists, testing the limits of how far carbon-based molecules can be distorted by combining them with metal atoms.
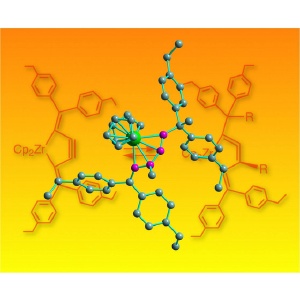 Pushing back the boundaries of chemistry. This molecular structure shows a zirconium atom (green) sitting in a ring with four other carbon atoms (purple) in the new cycloallene compound. In the background, the chemical reaction shows the interconversion between alkyne and allene.
Pushing back the boundaries of chemistry. This molecular structure shows a zirconium atom (green) sitting in a ring with four other carbon atoms (purple) in the new cycloallene compound. In the background, the chemical reaction shows the interconversion between alkyne and allene.
The complexity of organic chemistry is largely due to the tendency of carbon atoms to join together into chains and rings. Yet carbon–carbon triple bonds (–C=C–, also known as alkynes) are relatively rare in ring systems, because they prefer to connect to other atoms in straight lines.
It’s just about possible to bend this alkyne group so that it will fit into a ring of seven carbon atoms. But smaller rings are extremely reactive, due to the strain put on that triple bond, explains Noriyuki Suzuki of RIKEN’s Advanced Science Institute in Wako. “It was believed that it was impossible to isolate them in a pure form,” he says.
However, in 2002 Suzuki’s team reported that they had made a ring of just five atoms which included an alkyne group, yet was extremely stable and could be isolated as crystals. Their secret was that one of the atoms in the ring was a zirconium atom1.
The researchers are now exploring how this molecule behaves. Recently, they have used it to create another ring system containing two carbon–carbon double bonds sitting side by side (–C=C=C–), also known as an allene. Under normal circumstances a five-membered ring containing an allene would be too strained to exist for long—but with the zirconium atom in place, the team found that the cycloallene compound could be isolated.
The team made the compound by mixing lithium or potassium into a solution of their zirconium-alkyne ring, which gave it a negative charge and a deep blue color. Adding a reagent such as iodomethane then formed the cycloallene—although the team was surprised that adding a source of hydrogen atoms formed a mixture of a cycloallene and a cycloalkene, which contained just a single carbon–carbon double bond2.
Suzuki says that these compounds are unlikely to have any practical uses. Instead, the research pushes back the boundaries of chemistry that would once have been thought impossible. Suzuki notes that another group recently made a similar compound which included a hafnium atom3.
The team is now testing the physical and chemical properties of the new compounds, and trying to clarify exactly why these molecules are so stable.
- Suzuki, N., Nishiura, M. & Wakatsuki, Y. Isolation and structural characterization of 1-zirconacyclopent-3-yne, five-membered cyclic alkynes. Science 295, 660–663 (2002).
- Suzuki, N., Hashizume, D., Koshino, H. & Chihara, T. Transformation of a 1-zirconacyclopent-3-yne, a five-membered cycloalkyne, into a 1-zirconacyclopent-3-ene and formal “1-zirconacyclopenta-2,3-dienes”. Angewandte Chemie International Edition 47, 5198–5202 (2008).
- Ugolotti, J., Dierker, G., Kehr, G., Fröhlich, R., Grimme, S. & Erker, G. Five-membered metallacyclic allenoids: synthesis and structure of remarkably stable strongly distorted cyclic allene derivatives. Angewandte Chemie International Edition 47, 2622–2625 (2008).