Batteries are devices that store electrical energy by converting it to chemical energy, to be released slowly at a later time. In rechargeable batteries, this chemical process is reversible, allowing the battery to be reused many times.
Batteries are important to many areas of technology, including transport, portable electronics, medical devices, power tools, and storage of electricity produced by intermittent renewable sources (wind, solar, tidal, etc.).
Batteries are made from layers of different materials which enable the electrochemical storage of electricity - at a minimum, this will consist of an anode (positive electrode), a cathode (negative electrode) and electrolyte.
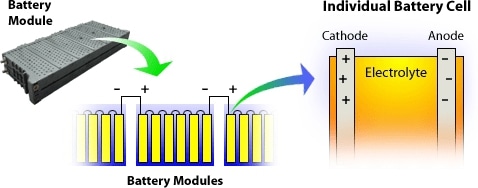
Figure 1. A simple schematic of a battery. Image Credits: NREL.gov
Battery technologies are continually improving, with ever-increasing energy densities and decreasing charge times. It is highly likely that the next major wave of innovations in batteries will take advantage of our increasing understanding of nanotechnology.
With our increasing use of high-performance batteries in hybrid and electric cars, and demanding electronic gadgets, the physical limits of the materials used in batteries are being tested. Enhancements through nanotechnology could provide a new lease of life for these materials, and also uncover new materials which have not yet been considered.
Benefits of Nanotechnology for Batteries
The main drivers for research in battery technology are to find materials suitable for use as electrodes with as high a surface area as possible. This allows charge to flow more freely, resulting in higher capacity and shorter charge/discharge cycles.
Safety of batteries is also an important concern. Liquid electrolytes, which are prevalent in Li-ion batteries, can rupture the cell and even combust when overheated. Currently, safety measures are required to prevent this from happening but take up space inside the battery, increasing its size, and adding cost and complexity to the manufacturing process.
These issues are being solved by nanotechnology research. Nanostructured materials can offer a huge step increase in surface area for electrolyte materials, and nanoparticles could enhance the conductivity of ceramics or gels sufficiently to allow them to replace liquid electrolytes, reducing or eliminating the chance of a short circuit.
Nanomaterials for Batteries
Electrodes
Several types of nanomaterials have been explored which allow for higher storage densities of lithium than standard metal or graphite electrodes:
- Carbon-coated silicon nanowires
- Carbon nanotubes
- Layered, nanostructured vanadium oxide and manganese oxide
- LiMn2O4 or LiCo2O4 nanoparticles
- Li alloy/Graphene foil
- Phosphorene-graphene hybrid material
Electrolytes
Al2O3, SiO2, or ZrO2 nanoparticles added to solid polymer gel could significantly enhance the conductivity and storage capacity of the electrolyte. Solid ceramics have also been explored, as their high-temperature resistance would suit demanding, high-stress applications like large vehicles or renewable power stations. 2D MoS2 is used as an efficient protective layer for Li metal anodes in high-performance Li–S batteries.
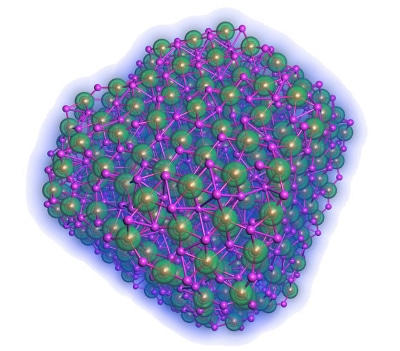
Figure 2. Nanoparticle matrices in battery electrodes can drastically increase their ability to store lithium ions, increasing the storage density of the battery. Image Credits: Argonne National Laboratory
Future Research for Nanotechnology in Batteries
Much of the research into nano-enhanced batteries in the coming few years will focus on reducing the cost of these nanomaterials, making them viable for large scale commercial applications.
The use of nanotechnology to enhance performance by increasing energy storage density has also allowed much smaller batteries to be made for applications which are less demanding but benefit from small, light and flexible rechargeable batteries. Some thin-film batteries are already available, but these have limited performance and are still relatively expensive. The advent of nano-based research and technology has improved the energy and power-density, cyclability and safety of modern batteries.
Sources and Further Reading
This article was updated on the 12th April, 2019.