AZoNano speak to Dr. Dominik Rejman about the innovative wound treatment, NANO-LLPO. This nanofibrous dressing can not only help to prevent bacterial infection but can promote wound healing as well. Read more about the research behind this exciting development, and how it is helping to advance the integration of nanotechnology in biomedicine.
Please could you introduce yourself and tell us what inspired your career in biotechnology research?
My name is Dominik Rejman, and I am a group leader at the Institute of Organic Chemistry and Biochemistry of the Czech Academy of Sciences, Prague (IOCB Prague). I graduated from the UTC Prague from organic chemistry and continued towards Ph.D. study at the IOCB Prague in the field of modified nucleosides, nucleotides, and oligonucleotides.
Since these molecules often possess fascinating biological properties, I have been connected with biochemistry, biology, and medicinal chemistry from the very beginning of my carrier. During my first postdoc position at the University of Sheffield, I worked on dinucleoside polyphosphates and their analogs. Then, I dealt with inhibitors of IMPDH and antiparasitic compounds in the Center for Drug Design at the University of Minnesota.
When I returned to Prague, my focus was aimed at antimicrobial compounds and, in general, new strategies to understand and treat infectious diseases.
How did you begin your involvement with nanomaterials?
Capitalizing on my antiparasitic compounds research, I have discovered, in collaboration with L. Krásný from the Institute of Microbiology and M. Kolář from the Palacký University Olomouc, novel antimicrobial compounds termed lipophosphonoxins (LPPO).
Following a lecture I gave on this subject, I was approached by the Head of Prague Burn Center R. Zajíček. He expressed his concern about infections in burn patients, and since he was involved in clinical trials of new nanomaterial fiber dressing NANOTARDIS developed in the Technical University, Liberec (TUL), we ended up with the idea of combining our molecules with the new nanomaterial.

Dominik Rejman / IOCB Prague. Image Credit: Tomas Bellon / IOCB Prague
Could you give an overview of your research?
I collaborated with professor D. Lukáš from TUL on this new composite material. First, we developed the fabrication method and then evaluated its antibacterial and mechanical properties. Finally, we performed the first in vivo experiment to validate its use in infectious wounds, which was quite successful.
NANO-LPPO is an exciting innovation in preventing skin infections as well as preventing bacterial strain resistance. Could you explain how this development compares to existing treatment methods?
The nanomaterial itself can promote wound healing, forming a sort of scaffold for cells to grow. An important property is also its biodegradability, mediated by enzymes lipases that are present in the wound.
When we combine the nanomaterial with our antibacterial LPPO, the LPPO is released mostly via biodegradation.
The presence of LPPO in the material increases the rate of degradation. This technology is particularly interesting because bacteria in the wound excrete the lytic enzymes and thus enhance biodegradation, releasing the antibacterial component LPPO.
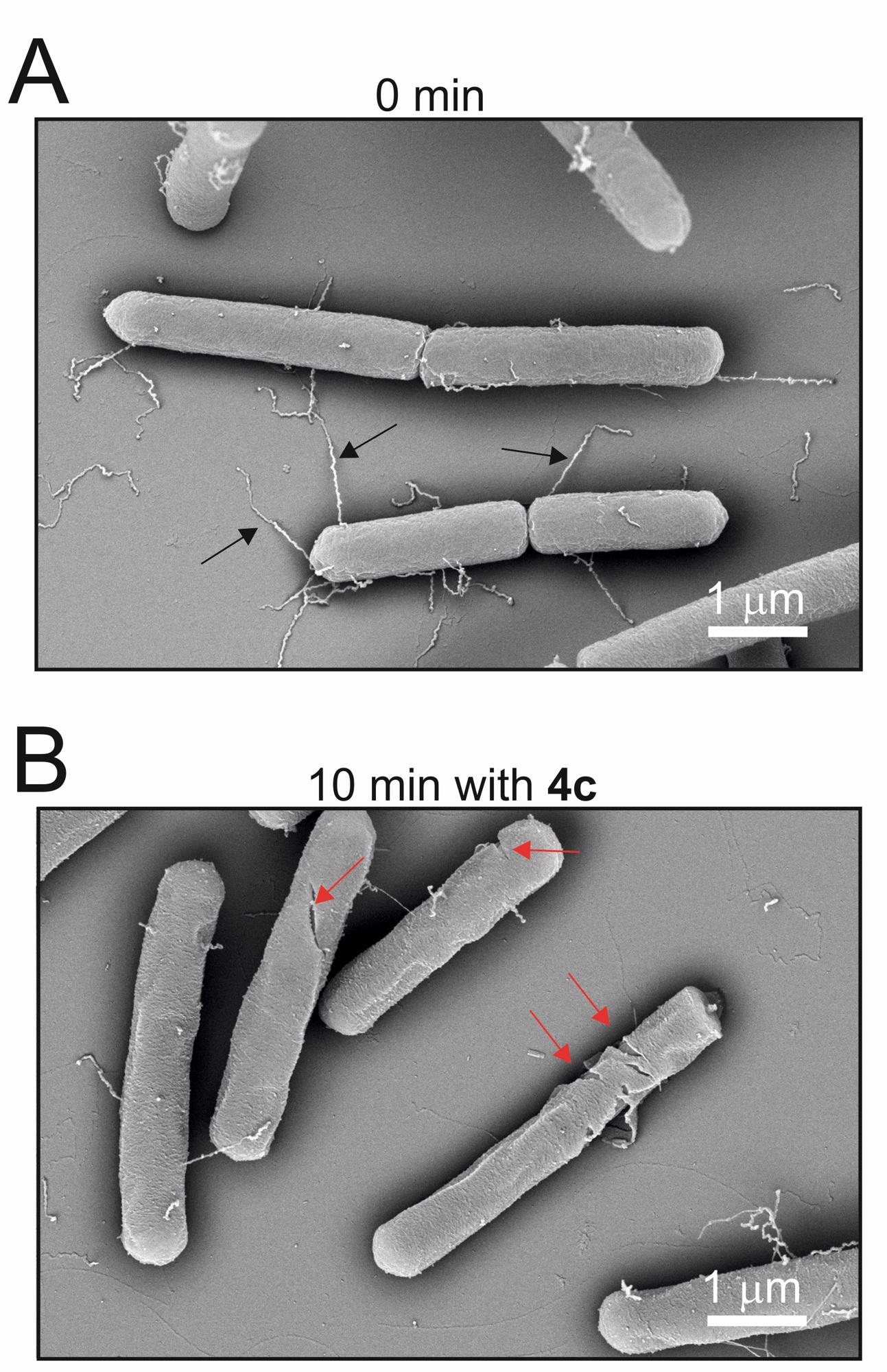
Bacillus subtilis bacteria before and after application of LPPO. The second image shows significant damage to bacteria after ten minutes. Image Credit: Institute of Microbiology of the CAS
In other words, the release of the antibacterial compound is partly controlled by the presence of the bacteria. Another important property is that the antibacterial component exerts its activity only at the site of infection. As it is not absorbed, it should not, in principle, form selection pressure for resistance development.
Moreover, we know it is very difficult for bacteria to develop resistance against LPPO – so far, we have been unable to select a resistant strain against LPPO, which is fantastic.
The effect of different concentrations of LPPO was tested on the nanofibrous wound dressings. How might different LPPO concentrations affect the ability of the nanofiber as a wound dressing?
The optimal LPPO concentration for the NANO-LPPO composite material needs to be addressed in the next experiments. So far, 5-10% seems to be sufficient to prevent infection in the wound, at least for S. aureus.
A key aspect of the methodology was defining the wettability of the wound dressing. What other parameters are important to address when designing active, biocompatible nanomaterials?
The wettability measurement was key to understanding why the presence of LPPO in the NANO-LPPO enhances its biodegradability. Other important parameters include the rate of degradation and the rate of LPPO release to the wound.
Another is the hydrophobicity of the material, which can be modified by the presence of the LPPO, as we showed, or by alteration of the polymeric composition of the nanomaterial itself.
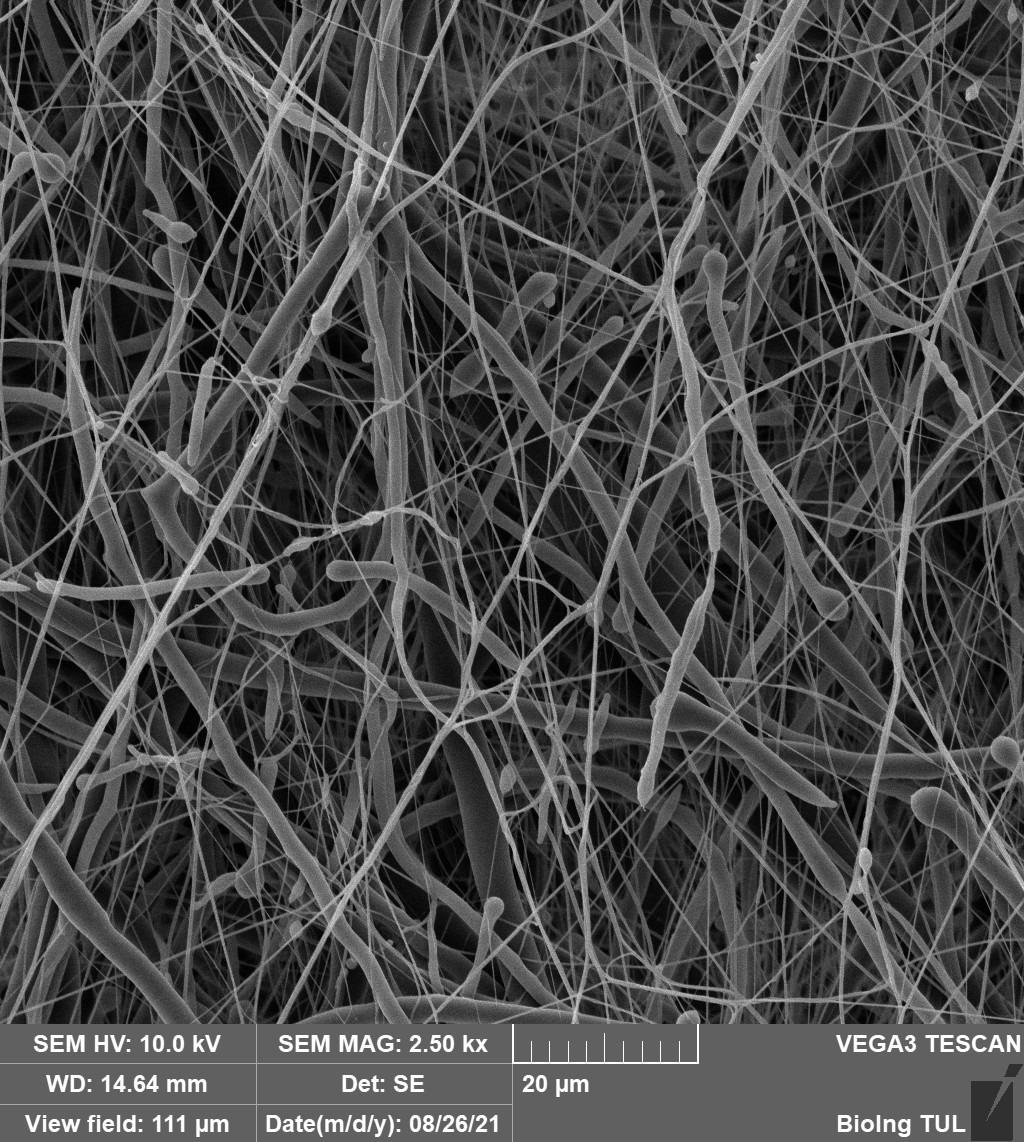
Nonwoven nanotextile NANOTARDIS. With its morphological and physical-chemical properties, the device promotes the healing of clean acute wounds. Image Credit: Technical University of Liberec
What major obstacles do you believe currently limit the involvement of nanofibers, and more broadly nanomaterials, for clinical applications?
I do not see any serious obstacle limiting the use of nanofibers. I see some concerns regarding the use of some nanoparticles due to their accumulation in tissues and potential chronic toxicity.
Infection treatment and promoting tissue regeneration are also important for internal damage. How could this research be extended to this application?
For internal use, where we can expect more systemic exposure to the antibacterial component, an antibacterial compound with extremely low systemic toxicity and minimal tendency to select for resistance is necessary. We hope this may be the next generation of LPPO, which we are currently working on.
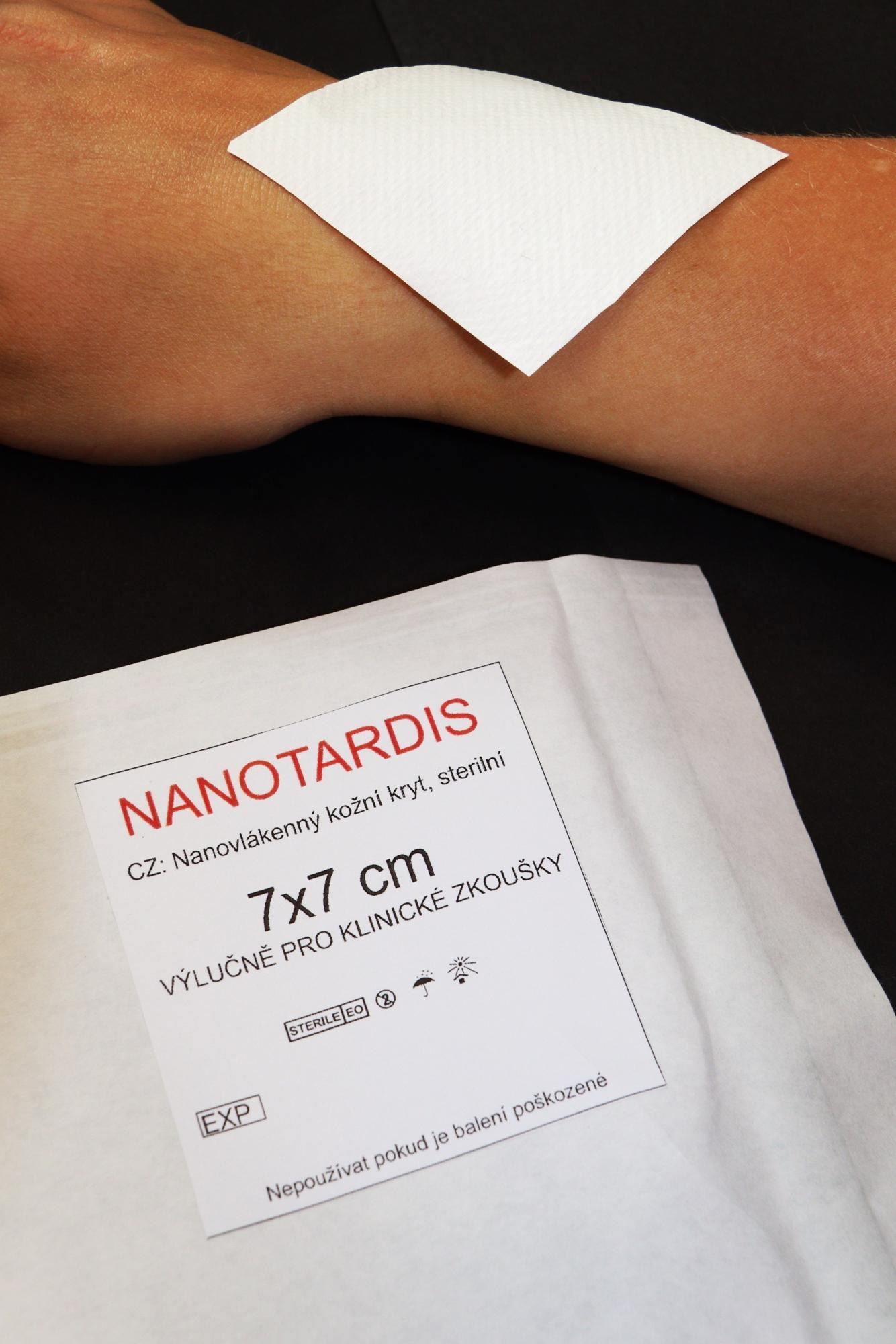
Nonwoven nanotextile NANOTARDIS. With its morphological and physical-chemical properties, the device promotes the healing of clean acute wounds. Image Credit: Technical University of Liberec
What are the next steps for this research, and more broadly the role of nanofibers and nanomaterials in clinical applications?
The next step in the research of NANO-LPPO will be the next phase of preclinical evaluation. This will involve a pig model, which is more relevant in terms of wound healing. When successful, then clinical evaluation can start.
I am optimistic about the use of modified nanofibrous materials in clinics, so I believe in 20 years, they will be a common tool in hospitals. I also expect that nanomaterial-based dressings for personal use will become commercially available.
Where can readers find more information on the progression of this research?
https://rejman.group.uochb.cz
https://www.iocbtech.cz/
Do Pham, D.D., et al. (2021) Novel lipophosphonoxin-loaded polycaprolactone electrospun nanofiber dressing reduces Staphylococcus aureus induced wound infection in mice. Sci. Rep. 11: 17688.
Látrová, K., Havlová, N., Večeřová, R., Pinkas, D., Bogdanová, K., Kolář, M., Fišer, R., Konopásek, I., Do Pham, D.D., Rejman, D., and Mikušová, G. (2021) Outer membrane and phospholipid composition of the target membrane affect the antimicrobial potential of first- and second-generation lipophosphonoxins. Sci. Rep. 11: 10446.
Seydlová, G., et al. (2017) Lipophosphonoxins II: Design, Synthesis, and Properties of Novel Broad Spectrum Antibacterial Agents. J. Med. Chem. 60: 6098-6118.
Zbornikova, E., et al. (2020) Evaluation of Second-Generation Lipophosphonoxins as Antimicrobial Additives in Bone Cement. ACS Omega 5: 3165-3171.
About Dr Dominik Rejman
 Dominik Rejman is a group leader at the Institute of Organic Chemistry and Biochemistry of the Czech Academy of Sciences, Prague (IOCB Prague), famous for Professor Antonín Holý‘s antiviral acyclic nucleoside phosphonates. Dr. Rejman graduated at UTC Prague from organic chemistry and continued his Ph.D. study at the IOCB Prague in the field of modified nucleosides, nucleotides, and oligonucleotides.
Dominik Rejman is a group leader at the Institute of Organic Chemistry and Biochemistry of the Czech Academy of Sciences, Prague (IOCB Prague), famous for Professor Antonín Holý‘s antiviral acyclic nucleoside phosphonates. Dr. Rejman graduated at UTC Prague from organic chemistry and continued his Ph.D. study at the IOCB Prague in the field of modified nucleosides, nucleotides, and oligonucleotides.
During his postdoc position at the University of Sheffield, Dr. Rejman focused his research on dinucleoside polyphosphates and their analogs. Next, he investigated inhibitors of IMPDH and antiparasitic compounds in the Center for Drug Design at the University of Minnesota. Following his return to Prague, Dr. Rejman’s focus was aimed at antimicrobial compounds and, in general, at new strategies to understand and treat infectious diseases.
Disclaimer: The views expressed here are those of the interviewee and do not necessarily represent the views of AZoM.com Limited (T/A) AZoNetwork, the owner and operator of this website. This disclaimer forms part of the Terms and Conditions of use of this website.