Solid lipid nanoparticles are considered to be at the forefront of the rapidly advancing field of nanotechnology, with applications within drug delivery, medicine, and research.
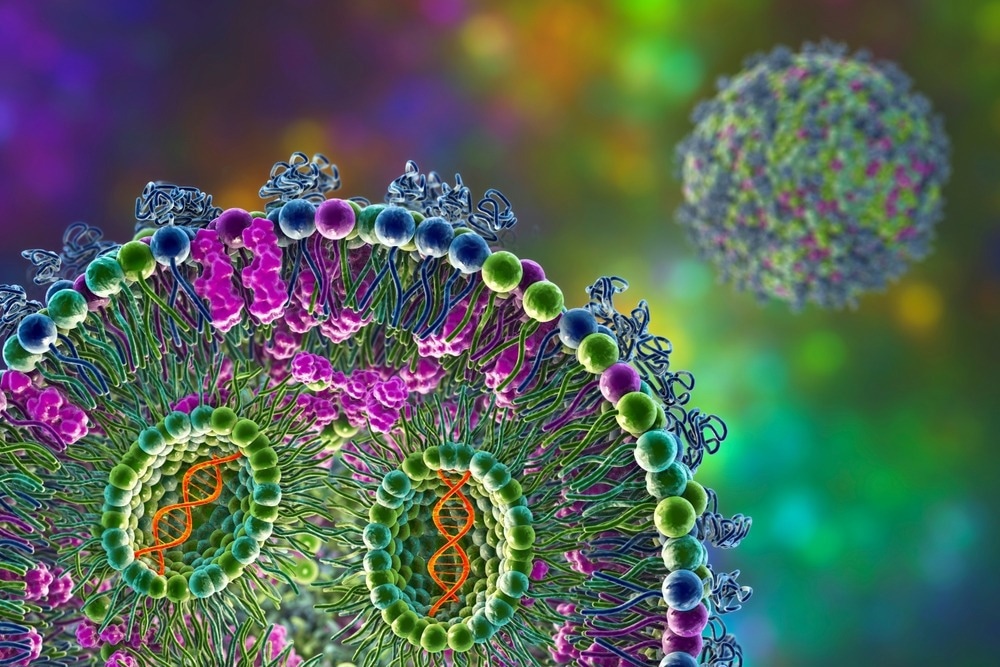
Image Credit: Kateryna Kon/Shutterstock.com
The unique physicochemical properties of these nanoparticles, dependent on their nanoscale size, enable this nanotechnology advancement to hold potential for novel development of therapeutics.
What Are Solid Lipid Nanoparticles?
Solid lipid nanoparticles (SLNs) can be described as colloidal drug carriers that have a diameter between 50 nm to 1 µm. These solid core lipid nanocarriers can hold both hydrophilic as well as hydrophobic drugs, making them a desirable choice for a drug delivery system.
The composition of solid lipid nanoparticles includes a physiological lipid that is either dispersed in water or within an aqueous surfactant solution. Other types of colloidal carriers within the category of nanolipid dispersions include but are not limited to liposomes, virosomes and ethosomes, with all of these making suitable delivery systems due to their biodegradability and non-toxic properties.
A distinguishing factor of solid lipid nanoparticles and nanostructured lipid carriers includes being more dominant and alterable to demonstrate varying benefits depending on the application, compared to liposomes and polymeric nanoparticles.
As displayed in Figure 1, solid lipid nanoparticles often have a spherical structure, with core ingredients for formulations comprising:
- Lipids, usually in a solid state at room temperature.
- Emulsifiers (or a combination of both).
- Active pharmaceutical ingredients.
- A solvent system.
Nanocarrier drug delivery systems can then be sub-categorized based on the route of administration, such as subcutaneous, and varying requirements of degradability. The ideal nanoparticle drug delivery system should typically have certain characteristics that elevate its desirability; this can aid researchers in developing successful drug delivery systems using solid lipid nanoparticles.
These features can include allowing for maximum drug availability and having precise targeting for diseased tissues, as well as having controlled drug release, allowing the drug delivery to be sustained for longer, and a minimal immune response.
Additionally, it should also be able to deliver drugs that are more conventionally difficult, including lipophiles and biomolecules, as well as having sufficient loading capacity of the drug active ingredient. Finally, there should also be good patient compliance, which is a significant component of any treatment.
Solid Lipid Nanoparticles: Benefits and Challenges
There are many advantageous features of solid lipid nanoparticles that have enabled this innovative development to spearhead the field of nanotechnology. Surface modification is considered a significant advantage of this drug delivery system, with all lipid nanoparticles having properties that can be enhanced and altered with surface modification, which can enable a higher level of drug targeting.
This holds significant implications for diseases such as cancer, where the use of a drug delivery system utilizing solid lipid nanoparticles can ensure only the diseased tissue is targeted, leaving the neighboring healthy tissues to be preserved. Subsequently, this can alleviate the toxicity associated with chemotherapy and reduce systemic toxicity, which can lead to organ failure and a poorer quality of life. Solid lipid nanoparticles can undergo surface modification with ligands that ensure they are targeted to the appropriate markers for a specific disease to ensure effective treatment.
Additionally, the stability of solid lipid nanoparticles has been described as more attractive than other nanoparticle formulations within literature due to aqueous solid lipid nanoparticles having the capacity to be stored for approximately up to 3 years and longer.
Subsequently, this drug delivery system, which utilizes solid lipid nanoparticles, can be more effective for commercial use as a result of the long shelf-life as well as effective drug release and bioavailability.
A study on pharmacokinetics has demonstrated the benefit of using solid lipid nanoparticles as a drug carrier, with an increase in the concentration of doxorubicin within nanoparticles compared to conventional commercial drug carriers.
The study also showed a higher concentration of the drug being found in different organs within rats, such as lungs, spleen and the brain with the use of solid lipid nanoparticles compared to conventional carriers.
The passing of the blood-brain barrier is promising for human application as brain cancer, known to be notoriously difficult to treat with low survival rates, may benefit from using solid lipid nanoparticles that could treat brain cancer and deliver effective anti-cancer drugs to the diseased tissue.
However, there are challenges with using solid lipid nanoparticles, including having poor drug loading capacity, which is limited by the solubility of the drug within the lipid melt, as well as other factors, such as the lipid matrix structure and the polymeric state of the matrix. Although this limitation is significant, it can be overcome by using more complex lipids in order to allow for a higher loading capacity.
Applications and Commercial Landscape of Solid Lipid Nanoparticles
Solid lipid nanoparticles can be used for different applications, including being used as a gene vector carrier, carrying either genetic or peptide materials such as DNA, plasmid DNA or other nucleic acids.
Gene therapy is a promising avenue of medicine that is being explored for the treatment of different diseases. The addition of nanotechnology in the form of solid lipid nanoparticles for gene therapy may aid in a significant advancement for the field, which was first provided with Food and Drug Administration (FDA) approval of a small interfering ribonucleic acid treatment (siRNA) using a lipid-based vector.
Another similar therapy that was approved in 2020 within the EU has shown promising results for treating hyperlipidemia, with this treatment demonstrating a 50% reduction in low density lipoprotein cholesterol levels in phase III clinical trials. The potential of solid lipid nanoparticles for various applications holds great potential for further FDA approvals.
Other applications of solid lipid nanoparticles can include being used in cosmeceuticals, which is considered to be the fastest growing sector of personal care, with solid lipid nanoparticles being applied to sunscreen formulations as a carrier for molecular sunscreens and UV blockers.
An in vivo study has demonstrated a 4% addition of solid lipid nanoparticles to conventional sunscreens can increase skin hydration by 31% after four weeks. With cosmeceuticals being a large and fast-growing sector, adding solid lipid nanoparticles to many personal care products may substantially transform this market and consumer satisfaction.
The global lipid nanoparticle market sales for 2023, including solid lipid nanoparticles, have been estimated to be US$ 887.2 million, with an estimated projected growth at a CAGR of 13.6% between the years 2023-2033, leading to sales of US$ 3,175.5 million by 2033, approximately.
With a competitive landscape for many prominent pharmaceutical companies that aim to utilize solid lipid nanoparticles for various applications, such as drug delivery, clinical research, and even cosmeceuticals, the growth rate of solid lipid nanoparticles can only be predicted to grow consistently and rapidly in the future. The translation of this projected forecast for lipid nanoparticles, including solid lipid nanoparticles in medicine, hold promise for a wide range of diseases, with researchers worldwide utilizing this technology to find effective treatments for patients.
References and Further Reading
Duan Y, Dhar A, Patel C, et al. (2020) A brief review on solid lipid nanoparticles: Part and parcel of Contemporary Drug Delivery Systems. RSC Advances. 10(45), pp. 26777-26791. https://pubs.rsc.org/en/content/articlelanding/2020/ra/d0ra03491f
Kaul S, Gulati N, Verma D, Mukherjee S, Nagaich U. (2018) Role of nanotechnology in Cosmeceuticals: A review of recent advances. Journal of Pharmaceutics. 2018, pp. 1-19. https://pubmed.ncbi.nlm.nih.gov/29785318/
Lipid nanoparticles market forecast 2023 to 2033: [Online] PharmiWeb.com.
Mukherjee S, Ray S, Thakur R. (2009) Solid lipid nanoparticles: A modern formulation approach in drug delivery system. Indian Journal of Pharmaceutical Sciences. 71(4), p. 349. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2865805/
Xu L, Wang X, Liu Y, Yang G, Falconer RJ, Zhao C-X. (2021) Lipid nanoparticles for Drug Delivery. Advanced NanoBiomed Research. 2(2), p. 2100109. https://onlinelibrary.wiley.com/doi/full/10.1002/anbr.202100109
Zu H, Gao D. (2021) Non-viral vectors in gene therapy: Recent development, challenges, and prospects. The AAPS Journal. 23(4). https://pubmed.ncbi.nlm.nih.gov/34076797/
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.