Sponsored by InovensoReviewed by Louis CastelAug 21 2023
Electrospinning offers a highly controllable and versatile approach for producing nanofibers with unique morphologies and properties which can be used in a wide range of applications in fields such as filtration, drug delivery, wound dressings, tissue engineering, customizable sensors, functional textiles, sound absorption, energy and electronics 1.
The superiority of electrospinning originates in the method allowing to tailor properties such as fiber diameter, porosity, surface area/volume ratio and other topographic features with the use of various natural, biosynthetic or petroleum-based polymers with respect to the application field 2.
However; the most commonly used solvents in this method include halogenated compounds (e.g. chloroform, dichloromethane, trifluoroethanol) and toxic solvents (e.g. dimethyl formamide, dimethyl acetamide, N-methyl pyrrolidone) due to their high solvation power for hydrophobic polymers. These types of solvents are either difficult to recycle or have high environmental impacts 3.
Concerning the commercialization of products using the electrospinning technique, many companies are hesitant to apply this method to their production lines because it requires significant know-how in solvent handling and a labored procedure to dispose of them properly 4.
Therefore; to make electrospinning more attractive as a commercial technique and to further improve scalability, solvent alternatives and other green pathways must be established that comply with social and legal regulations, particularly in terms of environmental and health impacts 5.
Many studies in the literature on classical solvent-based electrospinning showed that it is possible to spin almost all common polymers using alternative green solvents instead of toxic solvents. However, the rarity of published articles using greener solvent alternatives significantly reduces their visibility.
In this paper; the effects and strategies arising from using greener solvents for the production of electrospun fibers are emphasized to highlight ecofriendly alternatives of electrospinning technique with the aim of safety and overall sustainability of the electrospinning process and reduction of environmental impacts.
What is Green Solvent?
The definition of green solvents refers to the goal of minimizing the negative environmental impacts of the solvents, from chemical production to the disposal of the solvents.
There are many criteria to consider in order to label a solvent green or indicate how green it is as waste, environment, health and safety assessments 5.
The most comprehensive solvent guide has been prepared by GlaxoSmithKline (GSK); is given in Figure 1 below.
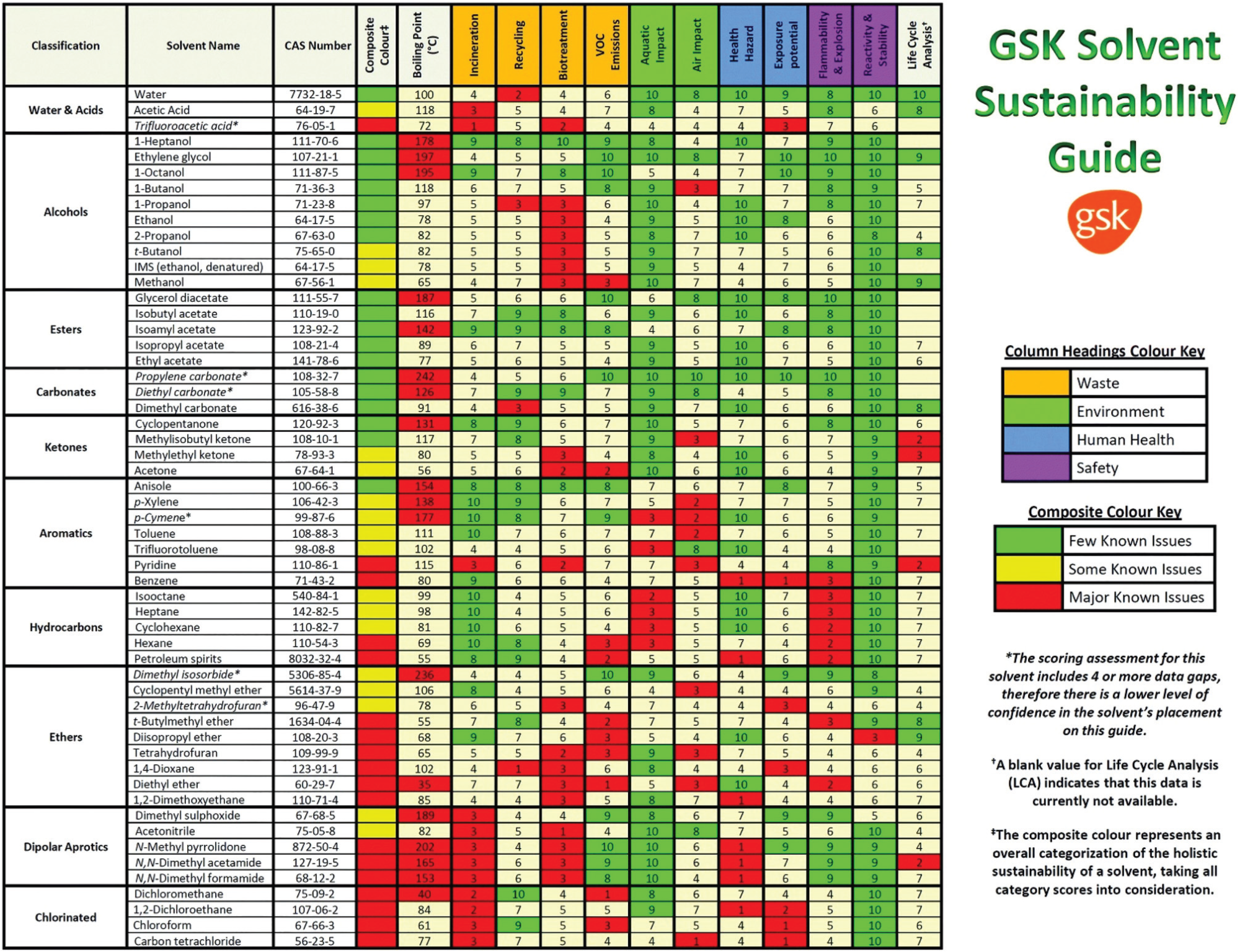
Figure 1. The GSK solvent sustainability selection guide showing the 55 most commonly used solvents scored and color-coded based on their greenness. In the ESI of ref. 29 is reported a chart classifying 154 solvents. Reproduced from ref. 5. Image Credit: Inovenso
In the table, the solvents are assigned by a value from 1 (the worst) to 10 (the best) for the categories of mainly sectioned as waste, environment, health, and safety divisions. “Waste” area is related to incineration, recycling, biotreatment, and volatile organic compound (VOC) emissions. “Environmental” area consists of air impact and aqueous impact. “Human Health” has 2 sections of potential of exposure and health hazard and “Safety” area is subtitled as flammability & explosion potential, and reactivity & stability respectively. And lastly the “composite score” gives an overall summary and is calculated as the geometric mean of each scores of the previous assessments.
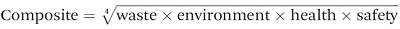
According to the solvent guide of GSK; the greenest solvents are DMSO, anisole, dimethyl propylene urea, formic acid, acetic acid, ketones, and alcohols. However, all the halogenated compounds are considered as not green 4.
Green Electrospinning
Green electrospinning refers to a more sustainable version of electrospinning that aims to reduce the environmental impact of the process. By using sustainable materials and processes, green electrospinning can help to reduce the carbon footprint and environmental impact of electrospinning while still producing high-quality fibers for a range of applications including biomedical, energy and environmental applications 5.
There are several ways to make electrospinning greener, such as using environmentally friendly solvents and/or replacing petroleum- based polymers with biodegradable or renewable materials. Below, there are some examples for the greener electrospinning of common industrial polymers used in certain fields in Table 1.
Table 1. Examples of electrospinning of widely used polymers using green solvents. Source: Inovenso
| Polymer |
Solvent/ solvent mixture |
Fiber Morphology |
Reference |
| CA |
AcOH: H2O 75:25 (w/w)
DMSO:Acetone 2:1 (v/v) |
Fibers 650 ± 130 nm |
6
7 |
| Chitosan |
AcOH:H2O 9:1 (w/w) |
130 nm |
8 |
| PA6 |
FA:H2O 85:15 (w/w)
AcOH:FA 3:1 (v/v) |
Nanofibers 124 nm |
9
10 |
| PA11 |
FA:Anisole 3:2 (v/v) |
53 ± 10 nm |
11 |
| PAN |
DMSO |
Nanofibers |
12 |
| PCL |
Acetone
FA |
Fibers
88 nm |
13
14 |
| PLA |
Acetone |
757 ± 275 nm |
15 |
| PMMA |
EtOAc
2-propanol :H2O 78:22 (w/w) |
Ribbon like microfiber
770 ± 45 nm |
16
17 |
| PS |
EtOAc / MEK
Limonene |
C-shaped Ribbon like 700 nm |
18
19 |
| PVDF |
DMSO:Acetone 3:2 (v/v) |
625 ± 113 nm |
20 |
| Zein |
EtOH:H2O 8:2 (v/v) |
126 ± 31 nm |
21 |
Cellulose Acetate (CA) is one of the chemically modified cellulose derivatives that could be used in a variety of industrial and food applications. CA could be dissolved in green solvents , such as water, methanol, EtOH or acetone and/or in acidic or alkaline conditions 6. Han et al has successfully electrospun CA using a binary solvent system of AcOH: H2O as 75:25 (w/w) ratio and obtained uniform bead-free nanofibrous structure. Haas and coworkers electrospun a solution consists of Acetone:DMSO (2:1 v/v) and obtained nanofibers without beads 7.
Chitosan (CS) is a deacetylated polysaccharide and a derivative of Chitin; mostly used in biomedical applications. Geng et al effectively electrospun CS nanofibers using an aqueous solution of 90% acetic acid. The average fiber diameter of the resulting membrane was 130 nm 8.
Polyamide 6 is one of the most common polymer used in the industrial applications and it is also soluble in green solvents like AcOH, FA, anisole and H2O etc. Supaphol and coworkers examined the effect of concentration of the polymer in the solution and obtained ultrafine nanofibrous mesh by using a solvent composition of FA: H2O (85:15 v/v) 9. Another study of De Vrieze et al. investigated the effect of the solvent ratios on the morphology and obtained ultrafine fibrous structure with a binary solvent composition of AcOH: FA (3:1 v/v) 10.
Polyamide 11 has been electrospun to produce another ultrafine nanofibrous structure by Meireman et al using a mixture of green solvents as FA: Anisole (3:2 v/v) 11.
Polyacrylonitrile (PAN) is a synthetic thermoplastic polymer used in the textile industry. Due to the chemical nature of PAN; there is only DMSO as a greener alternative to the toxic solvents of DMF or DMAc. Grothe et al worked on PAN dissolved in DMSO and obtained bead-free nanofibrous structure 12.
Polycaprolactone (PCL) is a synthetic, biocompatible and biodegradable polymer mostly used in the medical applications. Instead of popular toxic solvents like DMF or chloroform; PCL could also be dissolved in greener solvents ; such as AcOH, formic acid (FA) and acetone. Reneker et al used acetone for electrospinning of PCL and obtained uniform bead free nanofibers 13. In another study; Bahrami et al. examined different green solvent mixtures of formic acid, acetone, acetic acid and water. The solution containing formic acid with a 20% polymer concentration produced ultrafine nanofibrous structure. The 15% polymer solution prepared with acetic acid resulted with producing microfibers and the solution containing a binary solvent mixture of AcOH:FA (3:1 w/w) generates nanofibers with an average diameter of 266 nm 14.
Poly-Lactic Acid (PLA) is biodegradable, biocompatible and renewable thermoplastic polymer which is mainly derived from corn starch, potatoes, sugarcane and other biomass. PLA is a very useful candidate to be used instead of petroleum-based polymers due to its mechanical properties and easy processability. In one study; Casasola et al. has successfully electrospun 10% PLA in acetone and obtained bead-free nanofibrous structure 15.
Polymethyl Methacrylate (PMMA) is a transparent thermoplastic polymer which could be dissolved in many solvents like DMF, THF and chlorinated solvents. There are also greener alternative solvents for PMMA such as ethyl acetate (EtOAc), DMSO, 2- propanol, water and acetone. To give an example; Li and coworkers successfully electrospun 20% PMMA in ethyl acetate and get porous nanofibrous structure 16. Chang et al. also able to dissolve PMMA in 7.8:2 (w/w) 2-propanol:H2O mixture and effectively electrospun to produce bead free nanofibers 17.
Polystyrene (PS) is a well-known transparent polymer used in many different industrial applications. It is soluble in many solvents like DMF, THF, DMAc etc. Instead of toxic alternatives there are also green counterparts that could be used for the same purpose like EtOAc, limonene, methyl ethyl ketone (MEK), DMSO and acetone. Jarusawannapoom et al has been dissolved PS in MEK and EtOAc respectively to examine the process behavior with change of solvent. Both of the solvents produced similar outcomes as C shaped, ribbon like microfibers. It was interpreted that these results were due to the low boiling points of the solvents 18. Shin et al. obtained a successful nanofibrous membrane by using limonene as solvent for the solution 19.
Polyvinyledene Fluoride (PVDF) has fluorine atoms in its chemical structure, which gives the polymer a chemical inertness. Therefore; it could be dissolved using strong solvents like DMF. The only green alternative to DMF is DMSO and in the literature there are successful examples of electrospinning of PVDF using DMSO: Acetone mixtures. One of them is the study of Russo et al. to produce electrospun PVDF membranes using green solvents and she successfully obtain nanofibrous bead-free morphology using DMSO: Acetone as 3:2 (w/w) ratio.20
Zein is a vegetal protein extracted from renewable resources (mainly in corns) which is natural, biocompatible, and biodegradable. Zein is widely used in the pharmaceutical, biomedical, and packaging fields due to its hydrophobic and antibacterial properties. It could be dissolved in many green solvents like ethanol, acetic acid, formic acid etc and there are successful studies in the literature, giving promising examples of green electrospinnig of Zein. One of them has been employed by Neo et al and a bead free ultrafine fibrous structure has been obtained using the mixture of EtOH: H2O (8:2 w/w).21
Future Prospects for Green Electrospinning
Here are some present and future perspectives for green electrospinning:
- Sustainable Materials: Green electrospinning aims to use eco-friendly polymers, solvents, and additives to create nanofibers that are biodegradable, renewable, and non-toxic. Presently, there is a growing interest in using natural polymers, such as cellulose, chitosan, and proteins, as well as biodegradable synthetic polymers, such as polylactic acid (PLA) and polyhydroxyalkanoates (PHA), for electrospinning. Future perspectives involve further research and development of new sustainable materials that can be used for green electrospinning, including bio-based polymers and polymers derived from waste or renewable resources.
- Energy and Resource Efficiency: Green electrospinning aims to reduce the energy and resource consumption associated with the electrospinning process. Presently, efforts are being made to optimize the process parameters, such as voltage, flow rate, and distance between the needle and collector, to achieve maximum efficiency in terms of fiber production and energy consumption. Future perspectives involve the development of novel electrospinning techniques, such as needleless and near-field electrospinning, which can further improve energy and resource efficiency, as well as the integration of renewable energy sources, such as solar or wind energy, into the electrospinning process.
- Waste Reduction and Recycling: Green electrospinning also focuses on reducing waste and promoting recycling. Presently, efforts are being made to minimize waste generation during the electrospinning process, such as by using closed-loop systems to recover and reuse solvents, and by developing strategies to recycle electrospun fibers or products made from electrospun fibers. Future perspectives involve the development of biodegradable electrospun materials that can be easily recycled or composted, as well as the incorporation of waste-derived polymers or additives into the electrospinning process to promote circular economy principles.
- Applications in Sustainable Technologies: Green electrospun nanofibers have a wide range of potential applications in sustainable technologies. Presently, they are being used in areas such as biomedical and healthcare, environmental protection, and energy storage. For example, electrospun nanofibers can be used for drug delivery systems, wound dressings, water filtration, and electrochemical devices. Future perspectives involve exploring new applications and markets for green electrospun materials, such as in wearable devices, smart textiles, and sustainable packaging, as well as developing multi-functional electrospun materials with enhanced properties for specific applications.
- Standardization and Regulations: As green electrospinning gains momentum, there is a need for standardization and regulations to ensure the safety, quality, and sustainability of electrospun materials. Presently, efforts are being made to establish guidelines and standards for green electrospinning processes, materials, and products. Future perspectives involve further development and implementation of standards and regulations, as well as collaboration among researchers, industries, and policymakers to promote responsible and sustainable electrospinning practices.
In conclusion, green electrospinning presents promising present and future perspectives for creating sustainable materials with reduced environmental impact. Efforts are being made to develop eco-friendly materials, optimize energy and resource efficiency, reduce waste, explore new applications, and establish standardization and regulations. With continued research and innovation, green electrospinning has the potential to contribute to a more sustainable future.
References
- Figoli, A.; Simone, S.; Drioli, E. Hilal, N., Ismail, A.F.,Wright, C., Eds.; Taylor & Francis Group: Boca Raton, FL, USA, 2015; pp. 1–42.
- K. Ohmura, Nanofiber nonwoven fabrics by electrospinning and meltblown nonwovens, https://www.nonwovens-industry.com/issues/2018-11/view_far-east-report/nanofiber-nonwoven-fabrics-by-electrospinning-and-meltblown-nonwovens.
- Marino, T.; Galiano, F.; Simone, S.; Figoli, A. Environ. Sci. Pollut. Res. 2019, 26, 14774–14785.
- Avossa, J., Herwig, G., Toncelli, C., Itel, F., & Rossi, R. M. (2022). Green Chemistry, 24(6), 2347-2375. doi:10.1039/d1gc04252a
- Lv, D., Zhu, M., Jiang, Z., Jiang, S., Zhang, Q., Xiong, R., & Huang, C. (2018), 303(12), 1800336. doi:10.1002/mame.201800336
- S. O. Han, J. H. Youk, K. D. Min, Y. O. Kang and W. H. Park, Mater. Lett., 2008, 62, 759–762.
- D. Haas, S. Heinrich and P. Greil, J. Mater. Sci., 2010, 45,1299–1306.
- X. Geng, O. H. Kwon and J. Jang, Biomaterials, 2005, 26, 5427–5432.
- 55 P. Supaphol, C. Mit-Uppatham and M. Nithitanakul, J. Polym. Sci., Part B: Polym. Phys., 2005, 43, 3699–3712.
- S. De Vrieze, B. De Schoenmaker, Ö. Ceylan, J. Depuydt, L. Van Landuyt, H. Rahier, G. Van Assche and K. De Clerck, J. Appl. Polym. Sci., 2011, 119, 2984–2990.
- T. Meireman, L. Daelemans, S. Rijckaert, H. Rahier, W. Van Paepegem and K. De Clerck, Compos. Sci. Technol.,2020, 193, 108126.
- T. Grothe, T. Böhm, D. Wehlage and A. Remche, Tekstilec, 2017, 60, 290–295.
- D. H. Reneker, W. Kataphinan, A. Theron, E. Zussman and A. L. Yarin, Polymer, 2002, 43, 6785–6794.
- A. Gholipour-Kanani and S. H. Bahrami, J. Nanomater., 2011, 2011, 1–10.
- R. Casasola, N. L. Thomas, A. Trybala and S. Georgiadou, Polymer, 2014, 55, 4728–4737.
- H.-Y. Chang, C.-C. Chang and L.-P. Cheng, MATEC Web Conf., 2019, 264, 03004.
- T. Jarusuwannapoom, W. Hongrojjanawiwat, S. Jitjaicham, L. Wannatong, M. Nithitanakul, C. Pattamaprom, P. Koombhongse, R. Rangkupan and P. Supaphol, Eur. Polym. J., 2005, 41, 409–421.
- C. Shin and G. G. Chase, Polym. Bull., 2005, 55, 209–215.
- Russo, F., Ursino, C., Avruscio, E., Desiderio, G., Perrone, A., Santoro, S., . . . Figoli, A. (2020). Membranes, 10(3), 36. doi:10.3390/membranes10030036
- De Marco, I. (2022). Polymers, 14(11), 2172. doi:10.3390/polym14112172
- Y. P. Neo, S. Ray, A. J. Easteal, M. G. Nikolaidis and S. Y. Quek, J. Food Eng., 2012, 109, 645–651.

This information has been sourced, reviewed and adapted from materials provided by Inovenso.
For more information on this source, please visit Inovenso.