Aug 25 2009
The century-old challenge of transporting acetylene may have been solved in principle by a team of scientists working at the National Institute of Standards and Technology (NIST). A NIST research team has figured out* why a recently discovered material can safely store at low pressure up to 100 times as much of the volatile chemical as can be done with conventional methods.
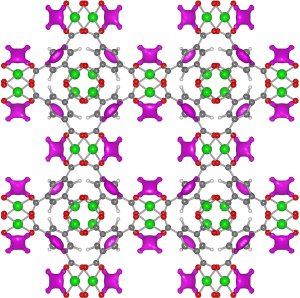 This closeup image of the HKUST-1 metal-organic framework (MOF), recently obtained by NIST scientists, reveals that copper atoms (green) are exposed to the open air within the MOF's lattice-like structure. The exposed copper allows the MOF to safely store acetylene (magenta) at up to 100 times current capabilities. Credit: Liu, NIST
This closeup image of the HKUST-1 metal-organic framework (MOF), recently obtained by NIST scientists, reveals that copper atoms (green) are exposed to the open air within the MOF's lattice-like structure. The exposed copper allows the MOF to safely store acetylene (magenta) at up to 100 times current capabilities. Credit: Liu, NIST
The team has probed the atomic-level workings of a metal-organic framework (MOF), a lattice-like structure made of copper oxide and benzene, that soaks up acetylene like a sponge. Using tools at the NIST Center for Neutron Research (NCNR), the scientists have shown that exposed copper atoms within the lattice give the MOF its talent at storing acetylene. The findings, according to NCNR physicist Yun Liu, could be of use to the chemical industry in the future.
“This discovery could provide substantial savings in acetylene transportation costs,” says Liu, a member of the research team, which also included scientists from the University of Texas at San Antonio.
Acetylene, widely used in decades past for welding and illumination, is now also valuable as a starting point for synthesizing a range of chemicals used in plastics and explosives. In the United States alone, several hundred thousand tons of acetylene are produced every year, but its volatility renders it difficult to transport: It becomes dangerously explosive at about 30 psi (207 kilopascal), only about twice normal atmospheric pressure. To safely store acetylene, storage cylinders have to be filled with both porous material and liquid solvents such as acetone.
The research team used neutron powder diffraction and computer calculations at the NCNR to investigate an MOF called HKUST-1, which has a sponge-like interior in which copper atoms are exposed to the air. The analysis showed that the acetylene attaches to the exposed copper by virtue of weak electrical charges, allowing the MOF to store 201 cubic centimeters of acetylene per gram of lattice at ambient pressure—comparable to the amount of similar chemicals that can be contained within a high pressure storage cylinder.
Liu says the fundamental discovery could also help scientists better understand MOFs, which could be used to store other materials. “More than a thousand of these metal—organic frameworks have been created so far,” he says. “We hope our technique will turn out to be a good way to check such materials’ properties in advance.”
* S. Xiang, W. Zhou, J.M. Gallegos, Y. Liu, and B. Chen. Exceptionally High Acetylene Uptake in a Microporous Metal – Organic Framework With Open Metal Sites. Journal of the American Chemical Society, Aug. 11, 2009, DOI 10.1021/ja904782h.