Sight would dramatically alter a blind man's understanding of an elephant, according to the old story. Now, a look directly at a cell surface is changing our understanding of cell membrane organization.
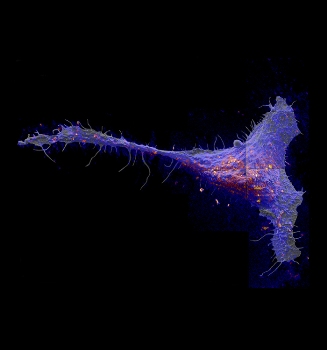 Researchers found that a class of molecules called sphingolipds congregate in large patches in the cell membrane. Red and yellow colors indicate local elevations in the sphingolipid abundance. (Photo by Kevin Carpenter)
Researchers found that a class of molecules called sphingolipds congregate in large patches in the cell membrane. Red and yellow colors indicate local elevations in the sphingolipid abundance. (Photo by Kevin Carpenter)
Using a completely new approach to imaging cell membranes, a study by researchers from the University of Illinois, Lawrence Livermore National Laboratory and the National Institutes of Health revealed some surprising relationships among molecules within cell membranes.
Led by Mary Kraft, a U. of I. professor of chemical and biomolecular engineering, the team published its findings in the Proceedings of the National Academy of Sciences.
Cells are enveloped in semi-permeable membranes that act as a barrier between the inside and outside of the cell. The membrane is mainly composed of a class of molecules called lipids, studded with proteins that help regulate how the cell responds to its environment.
"Lipids have multiple functions serving as both membrane structure and signaling molecules, so they regulate other functions inside the cell," Kraft said. "Therefore, understanding how they're organized is important. You need to know where they are to figure out how they're doing these regulatory functions."
One widely held belief among cell biologists is that lipids in the membrane assemble into patches, called domains, that differ in composition. However, research into how lipids are organized in the membrane, and how that organization affects cell function, has been hampered by the lack of direct observation. Although the cell membrane is heavily studied, the imaging techniques used infer the locations of certain molecules based on assumed associations with other molecules.
In the new study, Kraft's team used an advanced, molecule-specific imaging method that allowed the researchers to look at the membrane itself and map a particular type of lipid on mouse cell membranes. The researchers fed lipids labeled with rare stable isotopes to the cells and then imaged the distribution of the isotopes with high-resolution imaging mass spectrometry.
Called sphingolipids (SFING-go-lih-pids), these molecules are thought to associate with cholesterol to form small domains about 200 nanometers across. The direct imaging method revealed that sphingolipids do indeed form domains, but not in the way the researchers expected.
The domains were much bigger than suggested by prior experiments. The 200-nanometer domains clustered together to form much larger, micrometer-sized patches of sphingolipids in the membrane.
"We were amazed when we saw the first images of the patches of sphingolipids across the cell surface," said Peter Weber, who directed the team at Lawrence Livermore National Laboratory. "We weren't sure if our imaging mass spectrometry method would be sensitive enough to detect the labeled lipids, let alone what we would see."
Furthermore, when the researchers looked at cells that were low on cholesterol – thought to play a key role in lipid aggregation – they were surprised to find that the lipids still formed domains. On the other hand, disruption to the cell's structural scaffold seemed to dissolve the lipid clusters.
"We found that the presence of domains was somewhat affected by cholesterol but was more affected by the cytoskeleton – the protein network underneath the membrane," Kraft said. "The central issue is that the data are suggesting that the mechanism that's responsible for these domains is much more complicated than initially expected."
In addition, the new study found that sphingolipids domains were incompletely associated with a marker protein that researchers have long assumed dwelled where sphingolipids congregated. This means that data collected with imaging techniques that target this protein are not as accurate in representing sphingolipid distribution as previously thought.
"Our data are showing that if you want to know where sphingolipids are, look at the lipid, don't infer where it is based on other molecules, and now there's a way to directly image them," said Kraft, who also is affiliated with the department of chemistry at the U. of I.
Next, the researchers plan to use the direct-imaging method in conjunction with other more conventional methods, such as fluorescence, to further determine the organization of different kinds of molecules in the membrane, their interactions and how they affect the cell's function. They plan to begin by targeting cholesterol.
"Cholesterol abundance is important," Kraft said. "You change that, you tremendously change cell function. How is it organized? Is it also in domains? That's related to the question, what's the mechanism responsible for these structures and what are they doing?"